Volume 29, Number 4—April 2023
Dispatch
Two-Year Cohort Study of SARS-CoV-2, Verona, Italy, 2020‒2022
Abstract
We performed a follow-up of a previously reported SARS-CoV-2 prevalence study (April‒May 2020) in Verona, Italy. Through May 2022, only <1.1% of the city population had never been infected or vaccinated; 8.8% was the officially reported percentage. Limiting protection measures and vaccination boosters to elderly and frail persons seems justified.
In Italy at the beginning of 2022, a large part of the population >10 years of age was vaccinated against SARS-CoV-2 (1). Nevertheless, a high number of new infections occurred in the following months, largely caused by increasing contagiousness of new virus variants. Reliable data on the proportion of the population that remains naive (unvaccinated and no history of infection) are crucial to improve SARS-CoV-2 infection control policies. Relying only on reported cases caused a gross underestimation of the true prevalence in the early stages of the pandemic, both in Italy (2–5) and elsewhere (6–9).
In April and May 2020, at the end of the first pandemic wave in Italy, we performed a prevalence survey on a random sample in Verona, Italy, and showed that ≈3% of the population had acquired the infection, 5 times the official figures (4). We then performed a follow-up of this cohort, ending May 31, 2022, to monitor the cumulative incidence of the infection and to estimate the proportion of the city population that had never had the infection or had been vaccinated, thus remaining fully susceptible or naive.
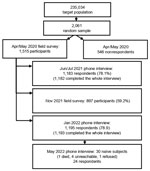
The study population has been described in detail (4). The initial cohort had 1,515 persons randomly selected from the city population (Figure 1; Appendix Figure). Mean age was 49.1 years, and most (54%) persons were women. Ten (0.7%) persons were positive for SARS-CoV-2 RNA, 40 (2.6%) were positive for IgG against nucleocapsid protein of SARS-CoV-2, and 1,465 (96.7%) tested negative. Using latent class analysis, we estimated a 3% prevalence of infection (4). We also summarize follow-up studies of the initial cohort (Figure 1).
We performed 3 follow-up surveys. The first survey was a telephone survey during June‒July 2021. The second survey, during November 2021, was in-person interviews on previous infections and vaccination status, and molecular (reverse transcription PCR) and antibody testing. The third survey was a telephone survey during January 2022.
On May 31, 2022, those persons who were still naive in January were interviewed again. Survey data were then compared with report data from the city’s health authority (Figure 2).
During June‒July 2021, of the initial cohort of 1,515 persons, 1,182 (78.0%) responded, of whom 134 (11.3%) reported having had SARS-CoV-2 laboratory-confirmed infection at least once. Of those who had been vaccinated (897, 75.9%), a total of 563 (62.8%) had already received the second dose. A total of 242 (20.5%) persons did not reported vaccination or previous infection.
During November 2021, a total of 897 persons (59.2% of the initial cohort) consented to participate. All were administered a questionnaire, and we obtained nasal and pharyngeal swab specimens and blood samples from all consenting persons. We performed reverse transcription PCR of swab specimens as described (4) to detect active infections; only 1 (0.1%) specimen showed a positive result.
We analyzed serum samples by using the SARS-CoV-2 IgG-N Assay (Abbott, https://www.ie.abbott) to detect IgG against nucleocapsid protein, as described (4). We also used the SARS-CoV-2 IgG II Quant Assay (Abbott) for the quantitative measure of IgG against spike (receptor-binding domain) protein according to the manufacturer’s procedure by using the ARCHITECT I System (Abbott). We also performed this test on biobank samples from the initial 2020 cohort, when the test was not yet available, to make it possible to compare 2020 results with 2021 results.
We compared the results of antibody tests from the survey with 2020 data (Table). A total of 160 (17.8%) of 897 persons tested positive for nucleocapsid IgG, (which is unaffected by vaccination), and 831 (92.7%) of 896 persons (1 missing value) tested positive for antibody against spike (receptor-binding domain) protein, which reacts to vaccination and natural infection. Of the 34 persons who had tested positive for nucleocapsid IgG during 2020, half had negative results at the following survey.
At the interview, of the 897 persons, 820 (91.4%) reported being vaccinated, of whom 735 (89.6%) had already received their second dose; 128 (14.3%) reported being infected at least 1 time. There were only 36 (4.0%) naive persons (no antibodies and no history of infection or vaccination).
During January 2022, of the 1,193 persons (78.7% of the initial cohort) who responded, 254 (21.3%) reported previous infections, and 1,123 (94.1%) had been vaccinated, including 322 (28.7%) with 2 doses and 764 (68.0%) with 3 doses. A total of 36 (3.0%) reported no infection or vaccination, of whom 6 were antibody positive in the previous survey. We classified the remaining 30 (2.5%) persons as naive and listed them for another interview on May 31, 2022.
During May 2022, of the 30 persons from the previous survey, 1 had died, 4 were unreachable, and 1 refused to answer. Of the remaining 24 persons, 8 were infected and remained unvaccinated, 2 were vaccinated and not infected, and 1 was vaccinated and infected. A total of 13 (1.1%) of 1,187 persons presumably remained naive. Thus, at the end May 2022, the best estimate from the study population was that 98.9% of persons >10 years of age in Verona had been infected, vaccinated, or both.
We compared data resulting from the random sample analysis (Figure 2) with reported data available up to May 31, 2022. The reported initial prevalence was much lower; the data had practically coincided in January 2022.
During May 2022 the cumulative reported incidence reached 31.2%, and the proportion of naive population was 8.8%, versus the 1.1% we found in our study (Figure 2). The actual percentage might be even lower, considering that results for IgG against nucleocapsid tend to become negative over time. Thus, we might have failed to detect some previous infections in the last field survey and, in any case, we did not perform further antibody investigations in the later stages.
According to the survey data, almost the entire population of Verona had some degree of protection in May 2022 against the severe forms of the disease. After a natural infection, the risk for severe forms of COVID-19 is much attenuated, even for nonvaccinated persons (10–13). The 3 doses of vaccine confer a long-term protection against severe disease, and hybrid immunity is more effective (14). Because immunity tends to wane over time, our finding that almost the whole population had been infected or vaccinated does not mean that all persons are protected. Moreover, immunity against new infections is much less effective and is short lasting, especially for the Omicron variants (11,15). The high contagiousness of these variants will predictably lead to continued circulation of the virus, which will act as a booster for most persons.
The first limitation of this study is that a not negligible proportion of the initial cohort were not able to be followed up. A selection bias cannot be excluded because persons who participated in the follow-up might be more health-conscious and more likely to adhere to vaccination. Only in November 2021 was it possible to repeat the molecular and serologic study. However, even with those limitations, we were able to reconstruct the trend of the pandemic in Verona and compare research results with reported data. Our estimate of the population that is still completely naive is lower than the official figures.
In Verona, the pandemic seems to have entered a phase in which we can be cautiously optimistic about its future course. It remains crucial to protect the frail and elderly persons, including those given booster vaccinations when indicated, but a cautious relaxation of restrictions for the general population seems justified, and repeated boosters for nonfrail persons might not be necessary.
Dr. Bisoffi is scientific director and director of the Department of Infectious, Tropical Diseases and Microbiology at the Istituto di Ricovero e Cura a Carattere Scientifico Sacro Cuore Don Calabria Hospital, Negrar di Valpolicella, Verona, Italy. He is the coordinator of TropNet, the European Network for Tropical Medicine and Travel Health. His primary research interests are surveillance and diagnosis of imported tropical and infectious diseases and clinical decision-making in tropical medicine.
Acknowledgments
We thank the study participants for their cooperation; the CEO of the Istituto di Ricovero e Cura a Carattere Scientifico Sacro Cuore Don Calabria Hospital Mario Piccinini for providing support; Stefano Tais, Monica Degani, and Eleonora Rizzi for coordinating laboratory work; Dora Buonfrate and Concetta Castilletti for critically reading the manuscript; hospital staff for providing assistance with swabs and blood sampling; and administrative staff for performing interviews.
This study was supported by the Italian Ministry of Health under Fondi Ricerca Corrente — Linea 1 and by European Union funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT).
References
- Task force COVID-19 del Dipartimento Malattie Infettive e Servizio di Informatica, Istituto Superiore di Sanità. COVID-19 epidemic. Country update: January 5, 2022 [in Italian] [cited 2023 Jan 19]. https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_5-gennaio-2022.pdf
- Albani V, Loria J, Massad E, Zubelli J. COVID-19 underreporting and its impact on vaccination strategies. BMC Infect Dis. 2021;21:1111. DOIPubMedGoogle Scholar
- Bassanello M, Pasini L, Senzolo M, Gambaro A, Roman M, Coli U, et al. Epidemiological study in a small rural area of Veneto (Italian region) during Sars-Cov-2 Pandemia. Sci Rep. 2021;11:23247. DOIPubMedGoogle Scholar
- Guerriero M, Bisoffi Z, Poli A, Micheletto C, Conti A, Pomari C. Prevalence of SARS-CoV-2, Verona, Italy, April-May 2020. Emerg Infect Dis. 2021;27:229–32. DOIPubMedGoogle Scholar
- Melotti R, Scaggiante F, Falciani M, Weichenberger CX, Foco L, Lombardo S, et al. Prevalence and determinants of serum antibodies to SARS-CoV-2 in the general population of the Gardena valley. Epidemiol Infect. 2021;149:
e194 . DOIPubMedGoogle Scholar - Angulo FJ, Finelli L, Swerdlow DL. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. JAMA Netw Open. 2021;4:e2033706–2033706. DOIPubMedGoogle Scholar
- Chamberlain AT, Toomey KE, Bradley H, Hall EW, Fahimi M, Lopman BA, et al. Cumulative incidence of SARS-CoV-2 infections among adults in Georgia, United States, August to December 2020. J Infect Dis. 2022;225:396–403. DOIPubMedGoogle Scholar
- Irons NJ, Raftery AE. Estimating SARS-CoV-2 infections from deaths, confirmed cases, tests, and random surveys. Proc Natl Acad Sci U S A. 2021;118:
e2103272118 . DOIPubMedGoogle Scholar - Maley JH, Sandsmark DK, Trainor A, Bass GD, Dabrowski CL, Magdamo BA, et al. Six-month impairment in cognition, mental health, and physical function following COVID-19‒associated respiratory failure. Crit Care Explor. 2022;4:
e0673 . DOIPubMedGoogle Scholar - Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22:781–90. DOIPubMedGoogle Scholar
- Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, et al. Protection against the Omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386:1288–90. DOIPubMedGoogle Scholar
- Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;386:2201–12. DOIPubMedGoogle Scholar
- Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804–16. DOIPubMedGoogle Scholar
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–44. DOIPubMedGoogle Scholar
- Suarez Castillo M, Khaoua H, Courtejoie N. Vaccine-induced and naturally-acquired protection against Omicron and Delta symptomatic infection and severe COVID-19 outcomes, France, December 2021 to January 2022. Euro Surveill. 2022;27:
2200250 . DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: March 14, 2023
1These first authors contributed equally to this article.
2These senior authors contributed equally to this article.
Table of Contents – Volume 29, Number 4—April 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Zeno Bisoffi, Istituto di Ricovero e Cura a Carattere Scientifico Sacro Cuore Don Calabria Hospital, Via Sempreboni 5, 37024 Negrar di Valpolicella, Verona, Italy
Top