Volume 29, Number 12—December 2023
Research Letter
Tuberculosis in Lemurs and a Fossa at National Zoo, Madagascar, 2022
Abstract
We diagnosed Mycobacterium tuberculosis in captive lemurs and a fossa in Antananarivo, Madagascar. We noted clinical signs in the animals and found characteristic lesions during necropsy. The source of infection remains unknown. Our results illustrate the potential for reverse zoonotic infections and intraspecies transmission of tuberculosis in captive wildlife.
In 2020, the World Health Organization estimated that in Madagascar there were 238 cases of Mycobacterium tuberculosis complex infection per 100,000 persons, but fewer than half of infected persons were given the appropriate diagnosis and notification from public health authorities. Madagascar healthcare and wildlife protection sectors have low efficacy because government programs and infrastructure often face insufficient funding, ineffective initiative implementation, and corruption (1).
Captured wild animals experience high levels of stress and are often malnourished, which leads to immunosuppression (2). Most of the zoos in Madagascar, including the country’s national zoo, the Botanical and Zoological Park of Tsimbazaza (PBZT), hold wild-captured wildlife and have substandard captive care compared with zoos in developed countries (3,4). Humans, who may harbor pathogens, and animals at captive facilities in Madagascar are in close contact, creating an ideal setting for zoonotic and reverse zoonotic disease transmission (5).
We previously reported M. tuberculosis (lineage 3 with streptomycin resistance) infection in a wild-captured, pet ring-tailed lemur (Lemur catta) in Madagascar (6). This disease has not been reported in wild lemurs, although few populations or species have been screened (7). In this report, we document M. tuberculosis infection in L. catta and several other threatened species of lemurs, as well as in a fossa (Cryptoprocta ferox), in Madagascar (Table; Appendix Table).
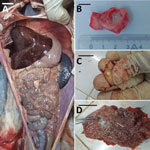
Animals that tested positive for M. tuberculosis (n = 10) were housed at the PBZT in Antananarivo, Madagascar. Infected animals displayed clinical signs for extended periods, including lethargy, anorexia, vomiting, and fever, leading to death. Necropsies of 4 animals revealed massive lymphohematogenous dissemination in black and white ruffed lemurs (Varecia variegata), including nodules, lesions, and white foci noted in lungs, kidneys, spleen, and mesentery (Figure). Caseous, inflamed, and necrotic lymph nodes, and hemorrhages were also present (Appendix Figure 1). Infected, living animals (n = 6) were sampled by using oropharyngeal swabs or bronchoalveolar lavage. No information regarding the presence of M. tuberculosis in PBZT staff could be obtained.
We confirmed M. tuberculosis infection by PCR on the clinical samples using the GeneXpert MTB/RIF Ultra assay (Cepheid, https://www.cepheid.com) and culture on BACTEC-MGIT liquid media (8). We conducted whole-genome sequencing on extracted DNA from culture isolates by using MinION (Oxford Nanopore Technologies; https://nanoporetech.com) and NovaSeq PE150 (Illumina, https://www.illumina.com/) sequencing platforms. We mapped decontaminated sequencing reads to the M. tuberculosis H37Rv reference genome (accession no. NC_000962.3). Lineage typing based on single-nucleotide polymorphisms revealed all isolates cluster to lineage 4.3.3. (Euro-American lineage, Latin American sublineage) (9) and have a maximum distance of 2 single-nucleotide polymorphisms (Appendix Figures 2, 3). GeneXpert MTB/RIF Ultra and sequencing resistance prediction from cultures of clinical isolates did not show any rifampin resistance.
We deposited all the sequence data used in this study into the National Center for Biotechnology Information Sequence Read Archive (accession no. PRJNA659624). We processed BACTEC-MGIT cultures at the National Reference TB Laboratory at the Institut Pasteur de Madagascar in Antananarivo. For every clinical isolate batch on BACTEC-MGIT culture, we used a positive-control mycobacteria growth indicator tube containing the laboratory reference stain h37Rv and a negative-control tube with the decontamination phosphate buffer. The National Reference TB Laboratory is externally certified twice annually for quality assurance and competency testing on BACTEC-MGIT culture.
Lineage 4 is both the most geographically widespread tuberculosis lineage and the most prevalent in persons residing in Antananarivo (10). Primates and other wildlife can contract Mycobacterium from humans (5). Given that the whole genome sequences from animals in the PBZT have a low maximum distance (2 single-nucleotide polymorphisms) and that the human isolates with the same sublineage L 4.3.3 were found in close proximity to the PBZT around the same time period, there is a possibility that an infected human transmitted the disease to multiple animals. However, it is also possible that interspecies transmission might have occurred, although it is not known whether lemurs or fossas are reservoir hosts for M. tuberculosis. If they are, transmission to wild lemurs and other endemic wildlife could pose a threat (6) because captive wild animals are sometimes released into forests or live near wild populations (2). Moreover, immunocompromised humans may be at risk for M. tuberculosis latent or active infection if they are close to diseased animals, such as those at PBZT.
The threat of TB transmission between humans and endangered wildlife, such as lemurs, invokes the need for changes that minimize interactions between humans and wildlife, reducing the chance of new disease outbreaks (5,6). Recommendations to protect Madagascar wildlife should include no wild capture of animals for captivity, no breeding of wildlife in substandard captive conditions, improved captive care that is comparable to international standards, and humane euthanasia of animals with communicable diseases or disease exposure. We also recommend annual testing (and negative results) for communicable diseases in humans who work in proximity to wildlife and no-contact restrictions for the public and wildlife. Thoese recommendations are consistent with the guidelines of the American Association of Zoo Veterinarians.
Dr. LaFleur is an associate professor at the University of San Diego, California, USA, and the founder and director of Lemur Love, a US-based nonprofit organization conducting research, conservation, and small-scale development in Madagascar. Her research examines the ecology of wild ring-tailed lemurs and the legal and illegal trades of wild-captured lemurs in Madagascar. She is additionally interested in animal welfare and zoonotic diseases of captive wildlife.
Acknowledgment
The University of San Diego provided ethical oversight (no. IACUC 0619–01).
References
- Bertelsmann Stiftung BTI. 2022 Country Report—Madagascar. Gütersloh: Bertelsmann Stiftung, 2022. [cited 2023 May 7]. https://bti-project.org/fileadmin/api/content/en/downloads/reports/country_report_2022_MDG.pdf
- Reuter KE, LaFleur M, Clarke TA, Holiniaina Kjeldgaard F, Ramanantenasoa I, Ratolojanahary T, et al. A national survey of household pet lemur ownership in Madagascar. PLoS One. 2019;14:
e0216593 . DOIPubMedGoogle Scholar - Ward SJ, Williams E, Groves G, Marsh S, Morgan D. Using zoo welfare assessments to identify common issues in developing country zoos. Animals (Basel). 2020;10:2101. DOIPubMedGoogle Scholar
- Berthine RA, Christine RA, Boromé RA. Bioclimatic of mycosis among wild and endemic animals captive-bred at Tsimbazaza Park in the Madagascar. Scientific Research Journal. 2018;VI:XI. DOIGoogle Scholar
- Chomel BB, Belotto A, Meslin FX. Wildlife, exotic pets, and emerging zoonoses. Emerg Infect Dis. 2007;13:6–11. DOIPubMedGoogle Scholar
- LaFleur M, Reuter KE, Hall MB, Rasoanaivo HH, McKernan S, Ranaivomanana P, et al. Drug-resistant tuberculosis in pet ring-tailed lemur, Madagascar. Emerg Infect Dis. 2021;27:977–9. DOIPubMedGoogle Scholar
- Honap TP, Pfister LA, Housman G, Mills S, Tarara RP, Suzuki K, et al. Mycobacterium leprae genomes from naturally infected nonhuman primates. PLoS Negl Trop Dis. 2018;12:
e0006190 . DOIPubMedGoogle Scholar - Ligthelm LJ, Nicol MP, Hoek KGP, Jacobson R, van Helden PD, Marais BJ, et al. Xpert MTB/RIF for rapid diagnosis of tuberculous lymphadenitis from fine-needle-aspiration biopsy specimens. J Clin Microbiol. 2011;49:3967–70. DOIPubMedGoogle Scholar
- Hunt M, Bradley P, Lapierre SG, Heys S, Thomsit M, Hall MB, et al. Antibiotic resistance prediction for Mycobacterium tuberculosis from genome sequence data with Mykrobe. Wellcome Open Res. 2019;4:191. DOIPubMedGoogle Scholar
- Ferdinand S, Sola C, Chanteau S, Ramarokoto H, Rasolonavalona T, Rasolofo-Razanamparany V, et al. A study of spoligotyping-defined Mycobacterium tuberculosis clades in relation to the origin of peopling and the demographic history in Madagascar. Infect Genet Evol. 2005;5:340–8. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: November 14, 2023
Table of Contents – Volume 29, Number 12—December 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Marni LaFleur, Department of Anthropology, University of San Diego, 5998 Alcala Park, San Diego, CA 92110-2492, USA
Top