Volume 12, Number 6—June 2006
Dispatch
Mixed Cryptosporidium Infections and HIV
Abstract
Mixed Cryptosporidium infections were detected in 7 of 21 patients with a diagnosis of rare Cryptosporidium canis or C. felis infections; 6 patients were infected with 2 Cryptosporidium spp. and 1 patient with 3 species. Mixed infections may occur more frequently than previously believed and should be considered when assessing cryptosporidiosis.
Cryptosporidium spp. infect humans and other vertebrate animals. Persons with compromised immune systems can suffer life-threatening chronic diarrhea, especially when their CD4+ lymphocyte counts fall <200 cells/μL. At least 7 Cryptosporidium spp. have been detected in immunocompromised patients (1). Nonetheless, the role of concurrent or mixed infections in the pathogenesis and transmission of Cryptosporidium spp. is unclear. Mixed infections of Cryptosporidium hominis and C. parvum have been reported in several patients from Switzerland and England (2,3). Additional studies from the United Kingdom reported simultaneous infections with these 2 species: 4 cases in 2 waterborne outbreaks and 2 cases of sporadic infections from 1995 to 1999 (4). In a more recent study, 12% of 135 clinical specimens from Aberdeenshire, Scotland, had concurrent C. parvum and C. hominis infections (5). Mixed C. hominis–C. parvum infections were also seen in 2 of 38 archived human specimens in a study conducted in the United States (6). These observations suggest that mixed Cryptosporidium infections are not uncommon.
Mixed infections may not be readily identified by commonly used molecular diagnostic tools because of preferential polymerase chain reaction (PCR) amplification of the predominant genotypes or the specificity of molecular tools (6). For example, PCR–restriction fragment length polymorphism (RFLP) tools based on the small subunit (SSU) rRNA gene are frequently used in genotyping Cryptosporidium spp. because they have higher sensitivity and detect more species than PCR-RFLP tools based on other genes (7).
Two previous studies in Peru used an SSU-rRNA–based PCR-RFLP tool to genotype Cryptosporidium specimens from children (8) and AIDS patients (1). A variety of Cryptosporidium spp. were found in both patient populations; C. hominis was the predominant species, followed by C. parvum, C. meleagridis, C. canis, and C. felis, but mixed infections were rarely detected (1,8). However, a recent study of some of the specimens that used PCR tools that selectively amplify DNA of C. parvum and closely related species identified concurrent infections of C. hominis in specimens previously diagnosed as having only C. canis, C. muris, or C. suis (7). Another recent study has shown that an SSU rRNA–based PCR-RFLP tool had only a 31%–74% success rate in detecting concurrent infections with C. parvum and C. hominis (9).
We addressed the question of whether Peruvian HIV-positive patients infected with the usual C. canis or C. felis parasites were co-infected with C. hominis, C. parvum, or C. meleagridis (7). The study protocol was approved by the participating institutional review boards. All participants gave written informed consent.
Mixed infections were identified by using 2 PCR-RFLP tools that only amplify C. hominis, C. parvum, or C. meleagridis (7). One tool was based on the dihydrofolate reductase (DHFR) gene (10) and the other on the Cryptosporidium oocyst wall protein (COWP) gene (11). Fifty-six stool specimens from 21 HIV-infected persons with previous diagnoses of C. canis or C. felis with an SSU rRNA–based PCR-RFLP tool were re-analyzed with these 2 molecular tools. DNA was extracted by using the QIAamp stool DNA extraction kit (Qiagen Inc., Valencia, CA, USA), and 1 μL DNA was used in nested PCR analyses of the DHFR and COWP genes. Secondary PCR products positive for Cryptosporidium were digested with restriction enzymes BpuA I for the DHFR tool or Rsa I for the COWP tool (10,11). Results of RFLP diagnosis were confirmed by DNA sequence analysis. All secondary PCR products were sequenced with a 3100 ABIPrism Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The sequences obtained were aligned with reference sequences from GenBank by using BioEdit version 7.0.5 (Isis Pharmaceuticals, Carlsbad, CA, USA).
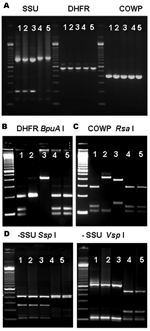
The PCR analysis of both DHFR and COWP genes showed that 17 specimens from 7 patients yielded products of the expected size for Cryptosporidium spp. (Figure, panel A, and Table). Restriction analysis of DHFR products with BpuA I showed that 4 patients had banding patterns indicative of C. hominis, 1 patient had the pattern of C. parvum, 1 patient had the pattern of C. meleagridis, and 1 patient had the patterns of C. hominis and C. meleagridis (Figure, panel B). Likewise, RFLP analysis of the COWP PCR products digested with Rsa I showed 3 banding patterns that were in complete agreement with the results obtained for the DHFR PCR-RFLP tool (Figure, panel C). Therefore, 2 of the 12 C. canis–infected patients had C. hominis, 1 had C. parvum and 1 had both C. hominis and C. meleagridis; of the 9 C. felis-infected patients, 2 had C. hominis and 1 had C. meleagridis (Table).
All DHFR and COWP PCR products were sequenced, which confirmed the results of the RFLP diagnosis. Altogether, 8, 2, and 3 DHFR sequences were obtained for C. hominis, C. parvum, and C. meleagridis, respectively. The C. hominis and C. meleagridis DHFR sequences were identical to XM_660774 and AY391725, respectively. The C. parvum DHFR sequences were homologous to XM_625460, with an insertion at position 37 and 4 bp substitutions at positions 66, 69, 364, and 367. Likewise, 10, 2, and 3 COWP sequences were obtained for C. hominis, C. parvum, and C. meleagridis, respectively, and were identical to AF481960, AF266273, and AY166840, respectively, in GenBank. The C. parvum DHFR nucleotide sequence obtained from this study is deposited in GenBank under accession no. DQ352814.
To confirm the original diagnosis of C. canis and C. felis infection, we reanalyzed all DNA preparations of these specimens with the SSU rRNA genotyping tool (7). Results were in complete agreement with those obtained previously (7): 19 specimens from 12 patients had C. canis, 15 specimens from 9 patients had C. felis, and no specimens had mixed Cryptosporidium spp., as indicated by RFLP patterns (Table and Figure panel D).
Data on diarrhea at study enrollment were available for 4 of the 7 patients with mixed infections and all 14 patients without mixed infections. Among persons with mixed infections, 1 did not have diarrhea, 2 had diarrhea lasting <30 days, and 1 had diarrhea >5 months. Seven of 14 patients without mixed infections had diarrhea: 5 had acute diarrhea lasting <30 days, and 2 had chronic diarrhea lasting >5 months (difference in prevalence of diarrhea for mixed versus single infections was not significant by the Fisher exact test). The average CD4+ lymphocyte count among the patients with mixed infections was 130 cells/μL. Of the 7 patients with mixed infections, 3 had specimens collected >30 days after the first detection, and mixed infections with the same species were still identified. The persistence of 2 species for >1 month is in contrast to a report that 1 Cryptosporidium genotype rapidly displaces the other during experimental infections of animals (6).
Concurrent infection with multiple Cryptosporidium spp. may affect clinical manifestations since C. hominis and C. parvum induce different sequelae in humans (12). The frequent finding of C. hominis in C. canis– and C. felis–infected persons also raises the question of infection sources. Although these 2 species are traditionally associated with animals, anthroponotic transmission may play a role in their acquisition in humans. Recent analyses demonstrate that a large proportion of human infections with C. parvum, another traditional zoonotic species, are actually due to anthroponotic transmission (13,14).
Our results also suggest that although the SSU rRNA–based PCR-RFLP tool or similar PCR techniques can detect and differentiate a wide range of Cryptosporidium species or genotypes, their usefulness in detecting mixed infections was compromised by preferential PCR amplification of the dominant species or genotype in specimens. This problem is likely inherited with most PCR tools. Thus, the use of PCR tools with broad specificity in combination with species-specific tools is needed to address the issue of mixed Cryptosporidium infections.
Our findings demonstrate that mixed infections are more frequent and persist longer in HIV-infected patients than previously believed. The clinical importance of these findings is not clear because of the study's cross-sectional nature. Future studies should employ tools that can detect mixed Cryptosporidium infections in longitudinal studies, evaluate the frequency of mixed infections of C. hominis and C. parvum, and assess their clinical and epidemiologic implications in both immunocompetent and immunocompromised persons.
Dr Cama is a researcher at the Centers for Disease Control and Prevention an an associate at Johns Hopkins University, Bloomberg School of Public Health. His current research interests include studies on the molecular epidemiology of enteric parasites that affect humans and have zoonotic potential.
Acknowledgment
This work was supported in part by funds from the Opportunistic Infections Working Group of the Centers for Disease Control and Prevention. Dr Gilman is supported by National Institutes of Health–National Institute of Allergy and Infectious Diseases grants Peru-TMRC 5P01AI051976-04 and R21 AI 059661-01.
References
- Cama VA, Bern C, Sulaiman IM, Gilman RH, Ticona E, Vivar A, Cryptosporidium species and genotypes in HIV-positive patients in Lima, Peru. J Eukaryot Microbiol. 2003;50(Suppl):531–3. DOIPubMedGoogle Scholar
- Fretz R, Svoboda P, Ryan UM, Thompson RC, Tanners M, Baumgartner A. Genotyping of Cryptosporidium spp. isolated from human stool specimens in Switzerland. Epidemiol Infect. 2003;131:663–7. DOIPubMedGoogle Scholar
- Gile M, Warhurst DC, Webster KA, West DM, Marshall JA. A multiplex allele specific polymerase chain reaction (MAS-PCR) on the dihydrofolate reductase gene for the detection of Cryptosporidium parvum genotypes 1 and 2. Parasitology. 2002;125:35–44.PubMedGoogle Scholar
- McLauchlin J, Amar C, Pedraza-Diaz S, Nichols GL. Molecular epidemiological analysis of Cryptosporidium spp. in the United Kingdom: results of genotyping Cryptosporidium spp. in 1,705 fecal specimens from humans and 105 fecal specimens from livestock animals. J Clin Microbiol. 2000;38:3984–90.PubMedGoogle Scholar
- Mallon M, MacLeod A, Wastling J, Smith H, Reilly B, Tait A. Population structures and the role of genetic exchange in the zoonotic pathogen Cryptosporidium parvum. J Mol Evol. 2003;56:407–17. DOIPubMedGoogle Scholar
- Tanriverdi S, Arslan MO, Akiyoshi DE, Tzipori S, Widmer G. Identification of genotypically mixed Cryptosporidium parvum populations in humans and calves. Mol Biochem Parasitol. 2003;130:13–22. DOIPubMedGoogle Scholar
- Jiang J, Xiao L. An evaluation of molecular diagnostic tools for the detection and differentiation of human-pathogenic Cryptosporidium spp. J Eukaryot Microbiol. 2003;50(Suppl):542–7. DOIPubMedGoogle Scholar
- Xiao L, Bern C, Limor J, Sulaiman I, Roberts J, Checkley W, Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis. 2001;183:492–7. DOIPubMedGoogle Scholar
- Reed C, Sturbaum GD, Hoover PJ, Sterling CR. Cryptosporidium parvum mixed genotypes detected by PCR-restriction fragment length polymorphism analysis. Appl Environ Microbiol. 2002;68:427–9. DOIPubMedGoogle Scholar
- Gibbons CL, Gazzard BG, Ibrahim MA, Morris-Jones S, Ong CS, Awad-El-Kareim FM. Correlation between markers of strain variation in Cryptosporidium parvum: evidence of clonality. Parasitol Int. 1998;47:139–47. DOIGoogle Scholar
- Pedraza-Diaz S, Amar C, Nichols GL, McLauchlin J. Nested polymerase chain reaction for amplification of the Cryptosporidium oocyst wall protein gene. Emerg Infect Dis. 2001;7:49–56. DOIPubMedGoogle Scholar
- Hunter PR, Hughes S, Woodhouse S, Raj N, Syed Q, Chalmers RM, Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin Infect Dis. 2004;39:504–10. DOIPubMedGoogle Scholar
- Alves M, Xiao L, Sulaiman I, Lal AA, Matos O, Antunes F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J Clin Microbiol. 2003;41:2744–7. DOIPubMedGoogle Scholar
- Mallon ME, MacLeod A, Wastling JM, Smith H, Tait A. Multilocus genotyping of Cryptosporidium parvum type 2: population genetics and sub-structuring. Infect Genet Evol. 2003;3:207–18. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 12, Number 6—June 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Lihua Xiao, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop F12, Atlanta, Georgia 30333, USA
Top