Volume 13, Number 2—February 2007
Dispatch
Postpartum Mastitis and Community-acquired Methicillin-resistant Staphylococcus aureus
Abstract
This single-center, case-control study documents a relative increase in methicillin resistance among 48 cases of Staphylococcus aureus–associated postpartum mastitis during 1998–2005. Of 21 cases with methicillin resistance, 17 (81%) occurred in 2005. Twenty (95%) isolates contained the Staphylococcus cassette chromosome mec type IV gene; this suggests that the increase is due to community-acquired methicillin-resistant Staphylococcus aureus.
Postpartum mastitis (PPM) occurs in as many as one third of breastfeeding women in the United States and leads to breast abscess formation in ≈10% of cases (1,2). Although breast milk cultures are not routine in PPM management, the growth of potentially pathogenic bacteria (such as β-hemolytic streptococci or Staphylococcus aureus) is associated with longer time to recovery and more frequent abscess formation (3). S. aureus is the most common bacterium isolated from such cultures, representing 37%–50% of isolates (4,5).
Reports of methicillin-resistant S. aureus (MRSA) PPM among young, healthy women lacking traditional risk factors for MRSA have emerged in the past few years (6,7). Isolates in these cases of community-acquired infection (CA-MRSA) remain susceptible to multiple non–β-lactam antibiotics and possess distinct molecular features (8).
Although risk factors associated with skin and soft tissue infections due to CA-MRSA have been described (8,9), characteristics unique to patients with CA-MRSA PPM are unknown. To identify risk factors, complications, and outcomes among patients with CA-MRSA PPM, we conducted a retrospective, case-control study to include all S. aureus–associated cases at a single institution over an 8-year period. MRSA isolates were analyzed by PCR for the presence of the Staphylococcus cassette chromosome (SCC) mec type IV gene, which is commonly associated with community-acquired infection.
We considered for analysis all patients from Northwestern University’s Prentice Women’s Hospital and affiliated Lynn Sage Comprehensive Breast Center with wound, fluid, drainage, or breast milk cultures positive for S. aureus from January 1998 through December 2005. Case-patients were defined as patients with PPM and a corresponding culture positive for MRSA. Control-patients were defined as patients with PPM and a corresponding culture positive for methicillin-susceptible S. aureus (MSSA). Patients who had no evidence of mastitis or who had a history of MRSA were excluded from the study. SCCmec types I–V were identified by a PCR-based multiplex assay; rapid bacterial DNA extraction and PCR amplification were performed as described elsewhere (10).
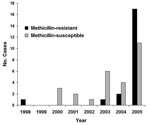
Forty-eight cases of S. aureus–associated PPM were identified during the study period; 21 cases were due to MRSA and 27 cases were due to MSSA. A relative increase in MRSA PPM was noted in the later years of the study (Figure 1, p = 0.04). MRSA and MSSA patients did not differ significantly with respect to age, pregnancy history, or symptoms at the time of initial evaluation. In addition, MRSA and MSSA patients did not differ in terms of potential risk factors for infection, such as diabetes, group B β-hemolytic streptococcus colonization, artificial rupture of membranes, epidural anesthesia, vaginal lacerations, episiotomy, cesarean section, or intrapartum antibiotic use (Table).
Ten (48%) MRSA and 11 (41%) MSSA patients required hospitalization. Although these inpatients did not differ in duration of symptoms before admission, length of stay, or leukocyte count, MRSA patients were more likely to have fever. One patient in each group required readmission for recurrent symptoms (Table).
Forty-six study patients had an abscess associated with mastitis; most (39 patients) underwent needle aspiration. Of these patients, 7 (41%) MRSA and 5 (23%) MSSA patients required repeat aspiration. Notably, 9 MSSA patients underwent incision and drainage a median of 4.5 days after aspiration (range 0–17 days), whereas only 1 MRSA patient required subsequent débridement (1 day later). Reasons for this difference are not clear; however, the more frequent use of serial ultrasound-guided aspiration in breast abscess management in recent years (when most MRSA cases occurred) may account for this finding.
In 17 of 21 MRSA cases, antibiotic use was documented. Twelve patients received antibiotics effective against MRSA, but only 2 received effective coverage at therapy onset (both received clindamycin). Patients initially received a penicillinase-resistant penicillin (10 patients), a first-generation cephalosporin (3 patients), a β-lactam/β-lactamase inhibitor (1 patient), or some combination of the above (6 patients). Median time to effective coverage for MRSA was 5 days (range 0–16 days); adequate antimicrobial agents included vancomycin (4 patients), trimethoprim-sulfamethoxazole (1 patient), clindamycin (9 patients), rifampin (2 patients), or some combination of the above (4 patients). Median duration of therapy, documented in 8 of 12 effective regimens, was 19 days (range 14–62 days).
Antimicrobial agent use was documented for 18 of 27 MSSA cases; in all 18 cases, isolates were susceptible to the initial antibiotic of choice. Initial regimens included penicillinase-resistant penicillins (10 patients), first-generation cephalosporins (2 patients), macrolides (1 patients), tetracyclines (1 patients), β-lactam/β-lactamase inhibitors (1 patient), vancomycin (1 patient), and clindamycin (2 patients). Duration of therapy for MSSA PPM, documented in 12 of 18 cases, was a median of 13.5 days (range 9–27 days).
Medical record review of affected patients did not show transmission of S. aureus to infants or other family members. In 1 MRSA patient, a perirectal abscess developed 5 months after the mastitis resolved. Intraoperative cultures of the abscess grew MRSA with identical susceptibilities, which suggests persistent colonization; however, typing of the isolates was not performed.
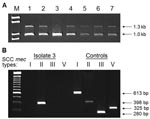
Of 21 MRSA isolates available for PCR analysis, 20 possessed SCC mec IV. The remaining isolate contained SCC mec II (Figure 2) and displayed resistance to clindamycin. In contrast, 95% of isolates with SCC mec IV were clindamycin susceptible.
To our knowledge, this is the largest case-control study of patients with MRSA-associated PPM. Although S. aureus is the most common etiologic agent of PPM, cases caused by MRSA have rarely been described. Epidemic MRSA cases, linked to the hospital transmission of a community-acquired isolate, have been observed more recently (6). Our study suggests that CA-MRSA is an increasingly common pathogen in spontaneous cases of PPM.
PPM due to CA-MRSA appears to be increasing at our institution. Among 17 MRSA-infected mothers in 2005, delivery dates spanned >9 months without overlap, which suggests that MRSA was independently acquired rather than outbreak-related. In addition, although isolates were not subjected to molecular typing by pulsed-field gel electrophoresis, PCR results suggest that 16 (94%) of MRSA isolates in 2005 were community-acquired.
The epidemiology of CA-MRSA PPM is poorly understood. Notably, nearly twice as many MRSA-infected than MSSA-infected women were multiparous in this study (57% vs. 33%, respectively). The prevalence of CA-MRSA is increasing among young children, and intrafamilial transmission of isolates has been documented (11,12); therefore, mothers with young children may be at increased risk for CA-MRSA PPM. Alternatively, these patients may serve as a reservoir for MRSA in the community, transmitting this organism to family members.
In the current study, women with MRSA were significantly less likely to receive adequate and timely antimicrobial drug treatment, but consequences of this difference are unclear. Lee et al. suggest that small CA-MRSA abscesses in children can be managed effectively with incision and drainage alone (13). Indeed, most women in this study underwent incision and drainage or wound aspiration without significant differences in outcomes. Although MSSA patients were more likely to undergo breast abscess incision and drainage than their MRSA counterparts, both methods are considered appropriate surgical interventions (14).
Although related cases of infant infection were not found, charts of household contacts were not reviewed in this study; cases of S. aureus transmission to infants or other family members may have been undetected. Several authors have reported mother-to-infant transmission of MRSA through breast milk (15,16). Although decolonization measures in MRSA-colonized patients have not demonstrated long-term effectiveness (17), the possibility of infant MRSA acquisition may warrant further evaluation of such measures in infected, breastfeeding mothers.
As with any retrospective case-control study, ours had several limitations. First, the study population is small, which limits the generalizability of the results. Second, patients were added to the study by using results of positive cultures; consequently, cases likely represented more severe and complicated infections in which cultures were necessary after routine therapeutic measures failed. Third, although PPM has been associated with multiple patient factors (i.e., difficulty breastfeeding, tobacco use, and stress), a thorough risk assessment is limited by retrospective study. In addition, medical record review may not indicate certain CA-MRSA risk factors, such as socioeconomic status, history of incarceration, or exposure to day care facilities. Finally, although the study results suggest a recent increase in MRSA PPM, an assessment of incidence would require further prospective analysis.
In summary, CA-MRSA has emerged as an increasingly common pathogen in PPM. Therapy against CA-MRSA should be considered in refractory or severe cases of PPM until wound, drainage, or breast milk cultures can be obtained. Adjunct surgical drainage or aspiration is often warranted in such cases. Additional study is required to determine the utility of routine cultures in postpartum mastitis, the prevalence of CA-MRSA in this emerging problem, and the consequences of CA-MRSA colonization for breastfeeding infants.
Dr Reddy is currently a fellow in the Division of Infectious Diseases at Northwestern University Feinberg School of Medicine. Her research and clinical interests include surgery-related infections and multidrug-resistant, nosocomial pathogens.
Acknowledgment
MSSA and MRSA isolates were identified from the microbiology database by Mike Malczynski. MRSA strains used as controls for SCCmec typing, including type I (NCTC10442), type II (N315), type III (85/2082), type IV (CA05), and type V (WIS [WBG8318]-JCSC3624), were kindly provided by Teruyo Ito. We also thank Patricia Garcia for her support and guidance.
References
- Barbosa-Cesnik C, Schwartz K, Foxman B. Lactation mastitis.JAMA. 2003;289:1609–13. DOIPubMedGoogle Scholar
- Foxman B, D’Arcy H, Gillespie B, Bobo JK, Schwartz K. Lactation mastitis: occurrence and medical management among 946 breastfeeding women in the United States.Am J Epidemiol. 2002;155:103–14. DOIPubMedGoogle Scholar
- Osterman KL, Rahm VA. Lactation mastitis: bacterial cultivation of breast milk, symptoms, treatment, and outcome.J Hum Lact. 2000;16:297–302. DOIPubMedGoogle Scholar
- Marshall BR, Hepper JK, Zirbel CC. Sporadic puerperal mastitis: an infection that need not interrupt lactation.JAMA. 1975;233:1377–9. DOIPubMedGoogle Scholar
- Niebyl JR, Spence MR, Parmley TH. Sporadic (non-epidemic) puerperal mastitis.J Reprod Med. 1978;20:97–100.PubMedGoogle Scholar
- Saiman L, O’Keefe M, Graham PLIII, Wu F, Said-Salim B, Kreiswirth B, Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women.Clin Infect Dis. 2003;37:1313–9. DOIPubMedGoogle Scholar
- Laibl VR, Sheffield JS, Roberts S, McIntire DD, Trevino S, Wendel GDJr. Clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus in pregnancy.Obstet Gynecol. 2005;106:461–5.PubMedGoogle Scholar
- Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection.JAMA. 2003;290:2976–84. DOIPubMedGoogle Scholar
- Charlebois ED, Perdreau-Remington F, Kreiswirth B, Bangsberg DR, Ciccarone D, Diep BA, Origins of community strains of methicillin-resistant Staphylococcus aureus.Clin Infect Dis. 2004;39:47–54. DOIPubMedGoogle Scholar
- Zhang K, McClure J, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of Staphylococcal cassette chromosome mec types I to IV in methicillin-resistant Staphylococcus aureus.J Clin Microbiol. 2005;43:5026–33. DOIPubMedGoogle Scholar
- Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposed risk.JAMA. 1998;279:593–8. DOIPubMedGoogle Scholar
- Jones TF, Creech CB, Erwin P, Baird SG, Woron AM, Schaffner W. Family outbreaks of invasive community-associated methicillin-resistant Staphylococcus aureus infection [cited 2006 Dec 26]. Clin Infect Dis [serial online]. 2006 Mar. Available from http://www.journals.uchicago.edu/CID/journal/issues/v42n9/38813/38813.web.pdf
- Lee MC, Rios AM, Aten MF, Mejias A, Cavuoti D, McCracken GHJr, Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus.Pediatr Infect Dis J. 2004;23:123–7. DOIPubMedGoogle Scholar
- Dener C, Inan A. Breast abscesses in lactating women.World J Surg. 2003;27:130–3.PubMedGoogle Scholar
- Kawada M, Okuzumi K, Hitomi S, Sugishita C. Transmission of Staphylococcus aureus between healthy, lactating mothers and their infants by breastfeeding.J Hum Lact. 2003;19:411–7. DOIPubMedGoogle Scholar
- Behari P, Englund J, Alcasid G, Garcia-Houchins S, Weber SG. Transmission of methicillin-resistant Staphylococcus aureus to preterm infants through breast milk.Infect Control Hosp Epidemiol. 2004;25:778–80. DOIPubMedGoogle Scholar
- Loveday HP, Pellowe CM, Jones SR, Pratt RJ. A systematic review of the evidence for interventions for the prevention and control of meticillin-resistant Staphylococcus aureus (1996–2004): report to the Joint MRSA Working Party (Subgroup A).J Hosp Infect. 2006;63(Suppl 1):S45–70. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 13, Number 2—February 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Pavani Reddy, Division of Infectious Diseases, 676 N Saint Clair St, Suite 200, Chicago, IL 60611, USA;
Top