Volume 13, Number 8—August 2007
Dispatch
Migrating Birds and Tickborne Encephalitis Virus
Abstract
During spring and autumn 2001, we screened 13,260 migrating birds at Ottenby Bird Observatory, Sweden, and found 3.4% were infested with ticks. Four birds, each a different passerine species, carried tickborne encephalitis virus (TBEV)–infected ticks (Ixodes ricinus). Migrating birds may play a role in the geographic dispersal of TBEV-infected ticks.
Tickborne encephalitis is a viral zoonotic disease caused by the tickborne encephalitis flavivirus (TBEV). There are 3 subtypes of TBEV: the European subtype (TBEV-Eu, transmitted by Ixodes ricinus ticks) and the Siberian and Far-Eastern subtypes (TBEV-Sib and TBEV-FE, transmitted by I. persulcatus ticks) (1–3). Geographic distribution of TBEV subtypes largely follows that of their tick hosts: I. ricinus (Europe) and I. persulcatus (from Far East to the Baltic countries) (4). In Latvia and Estonia, the distribution of both tick species overlaps, and all 3 TBEV subtypes cocirculate in Latvia (3). Thus, a range expansion of a tick species could result in spreading a TBEV subtype to new areas.
Small rodents are thought to be the main amplifying hosts, although wild ungulates contribute indirectly by providing blood meals for adult ticks, thereby maintaining the vector populations necessary for virus transmission. In addition to mammals, I. ricinus ticks take blood meals from birds, which has led to speculation that birds could disperse TBEV-infected ticks during migration and start new TBE foci. In this study, we document the occurrence of TBEV-infected ticks in migrating birds.
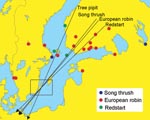
Fieldwork was conducted during 2001 at Ottenby Bird Observatory, located on the southernmost tip of Öland, a large island off the southeast coast of Sweden (56° 12′ N, 16° 24′ E; Figure). Throughout spring (March 25–June 15) and autumn (July 1–November 15) migration, observatory personnel captured and screened birds for ticks, except during 8 days when an excessive number of trapped birds made complete monitoring impossible. Each captured bird was identified by species and age and was banded. For bird species with TBEV-infected ticks, local banding and recovery records from 1946 to the present were used to determine recruitment and wintering areas.
Tick screening comprised rapid visual assessment for the presence of any ticks on bare body parts, especially around the eyes and beak of each bird. All ticks were removed by forceps, placed separately into snap-lid tubes, frozen and stored at –70οC, and then analyzed with a dissecting microscope to identify species and development stage.
A Puregene RNA isolation protocol adopted for 100–10,000 cells (Gentra Systems, Minneapolis, MN, USA) individually homogenized each tick and extracted RNA, according to the manufacturer’s instructions. The RNA pellet was resolved in 25 µL DNA hydration buffer and stored at –70°C until further analysis.
Samples were pooled 10 by 10 (5 µL from each individual extract) and analyzed by a nested reverse transcription (RT)-PCR targeting the 5′-terminal noncoding region (5) for the initial detection of TBEV RNA. Briefly, the RT-PCR was performed in 25-µL reaction volumes containing 1× EZ buffer, 0.3 mmol of each deoxyribonucleotide (dNTP), 2.5 U rTth DNA polymerase, 2.5 mmol Mn(OAc)2 (all reagents provided from Perkin Elmer, Branchburg, NJ, USA), 25 pmol of each primer (Pp1 and Pm1), 25 U Rnasine (Gibco, Paisley, Scotland, UK), and the target viral RNA. The reaction was performed in a GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) programmed to incubate 45 min at 60°C for RT and 2 min at 94°C for denaturation as initial steps, followed by 40 cycles of 30 s at 94°C and 30 s at 66°C. The final extension was for 5 min at 66°C. Negative and positive controls were included in each PCR run.
A second amplification step was conducted with 2 µL of the first amplification products. The total reaction volume of 25 µL included 1× PCR buffer II, 1.5 mmol MgCl2, 0.2 mmol each of dNTP, 0.625 U AmpliTaq Gold polymerase (Perkin Elmer), and 25 pmol of each internal primer (Pp2 and Pm2). After a pre-incubation step of 9 min at 95°C, the reaction was continued by 30 cycles of 15 s at 94°C and 30 s at 65°C and ended with an elongation step of 10 min at 72°C. Samples from positive pools were rerun using individual samples with the nested PCR described above.
During the study period, 1,155 ticks were collected from 447 (3.4%) of 13,260 screened birds (Table). Nearly all ticks (1,130) were reliably identified as I. ricinus. Seven nymphs showed characters resembling I. lividus, but these and 19 other ticks were rather poorly preserved, making identification uncertain. Frequencies of the various tick life stages were as follows: larvae (53.4%), nymphs (45.1%), and adults (0.6%). The mean infestation rate (0.086 immature ticks per examined bird, 2.6 immature ticks per infested bird) was unevenly distributed among bird species, with tick infestation in only 37 of >100 investigated species.
Ground-foraging birds carried ≈80% of all detected ticks and made up 71.3% of all infested birds (Table). A few ticks were also found on granivorous bird species, e.g., siskins, finches, sparrows, and some insectivorous songbirds, particularly among Sylvia and Acrocephalus warblers that forage in reed beds or dense stands of herbaceous plants (Table). The number of detected ticks per infested bird was usually in the range of 1–5 ticks, but 2 birds, a song thrush (Turdus philomelos) and a European robin (Erithacus rubecula), carried 41 and 39 ticks, respectively.
After initial screening of pools and rerunning individual samples from PCR-positive pools, we detected 6 TBEV-positive samples: 4 tick nymphs and 2 larvae. One larva was collected from a juvenile tree pipit (Anthus trivialis), 1 nymph each from a song thrush and juvenile redstart (Phoenicurus phoenicurus), and 2 nymphs and 1 larva from a juvenile European robin. All TBEV-infected ticks were collected from birds during the autumn migration. Despite repeated trials, we were unable to obtain readable sequence data from the positive samples and could not identify the TBEV strains by subtype.
Our study found that some ticks attached to birds carried TBEV. However, the frequency of TBEV among such ticks was less than the frequency of Borrelia burgdorferi senso lato from similar datasets (6–8). Analyses of banding recovery data for the 4 bird species with TBEV-infected ticks indicate an eastern recruitment area coinciding with TBE-endemic areas in Fennoscandia and western Russia (Figure).
TBEV has been isolated, or serologically indicated, from several bird species, especially anatids and gallinaceous birds, and most often from Eastern Europe or Russia (9). However, little is known about the capability of birds to function as reservoirs of TBEV, and small rodents remain the most important reservoirs of the virus. The fact that we found 2 I. ricinus larvae infected with TBEV could indicate that these birds may be reservoirs, because these larvae did not feed before attaching themselves to the birds. However, nonviremic transmission between ticks cofeeding on the same host has been shown to occur with TBEV (10) and other arboviruses (11,12), and we did not look for viremia in the tick-infested birds.
The migration of birds through Scandinavia during spring and fall involves several hundred million birds. Although the tick infestation rate per bird was not great in our study, and TBEV-infected ticks were only a small fraction of all ticks, the vast numbers of migrating birds do increase the probabilities for geographic spread of ticks and TBEV, in particular for TBEV-Eu, because I. ricinus predominated in our sample. Our data add to the growing body of evidence showing that migratory birds can disperse ticks infected with medically important pathogens (6,7,10,13).
Dr Waldenström is assistant professor at the Section for Zoonotic Ecology and Epidemiology, Kalmar University, Sweden. He has a broad interest in birdborne zoonotic infections from ornithologic and epidemiologic perspectives.
Acknowledgments
We thank staff and volunteers at Ottenby Bird Observatory for assistance with collecting ticks.
This work was supported by the Swedish Medical Research Council (07922) and the Swedish Council for Forestry and Agricultural Research (grant no. 23.0161). This is contribution no. 217 from Ottenby Bird Observatory.
References
- Ecker M, Allison SL, Meixner T, Heinz FX. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol. 1999;80:179–85.PubMedGoogle Scholar
- Mavtchoutko V, Vene S, Haglund M, Forsgren M, Duks A, Kalnina V, Characterization of tick-borne encephalitis virus from Latvia. J Med Virol. 2000;60:216–22. DOIPubMedGoogle Scholar
- Lundkvist Å, Vene S, Golovljova I, Mavtchoutko V, Forsgren M, Kalnina V, Characterization of tick-borne encephalitis virus from Latvia: evidence for co-circulation of tree distinct subtypes. J Med Virol. 2001;65:730–5. DOIPubMedGoogle Scholar
- Monath TP, Heinz FX. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology, 3rd ed. Philadelphia: Lippincot-Raven Publishers; 1996. p. 961–1034.
- Schrader C, Süss J. A nested RT-PCR for detection of tick-borne encephalitis virus (TBEV) in ticks in natural foci. Zentralbl Bakteriol. 1999;289:319–28.PubMedGoogle Scholar
- Comstedt P, Bergström S, Olsen B, Garpmo U, Marjavaara L, Mejlon H, Migratory passerine birds as reservoirs of Lyme borreliosis in Europe. Emerg Infect Dis. 2006;12:1087–95.PubMedGoogle Scholar
- Olsen B, Jaenson TGT, Bergström S. Prevalence of Borrelia burgdorferi sensu lato–infected ticks on migrating birds. Appl Environ Microbiol. 1995;61:3082–7.PubMedGoogle Scholar
- Marie-Angèle P, Lommano E, Humair PF, Douet V, Rais O, Schaad M, Prevalence of Borrelia burgdorferi senso lato in ticks collected form migratory birds in Switzerland. Appl Environ Microbiol. 2006;72:976–9. DOIPubMedGoogle Scholar
- Hubálek Z. Pathogenic microorganisms associated with free-living birds (a review). Acta Sc Nat Brno. 1994;28:1–74.
- Labuda M, Nuttal PA, Kožuch O, Elečková E, Williams T, Žuffová E, Non-viraemic transmission of tick-borne encephalitis virus: a mechanism for arbovirus survival in nature. Experentia. 1993;49:802–5. DOIGoogle Scholar
- Jones LD, Davies CR, Steele GM, Nuttal PA. A novel mode of arbovirus transmission involving a nonviremic host. Science. 1987;237:775–7. DOIPubMedGoogle Scholar
- Norman R, Ross D, Laurenson MK, Hudson PJ. The role of non-viraemic transmission on the persistence and dynamics of a tick borne virus—Louping ill in red grouse (Lagopus lagopus scoticus) and mountain hares (Lepus timidus). J Math Biol. 2004;48:119–34. DOIPubMedGoogle Scholar
- Bjöersdorff A, Bergström S, Massung RF, Haemig PD, Olsen B. Ehrlichia-infected ticks on migrating birds. Emerg Infect Dis. 2001;7:877–9. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 13, Number 8—August 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jonas Waldenström, Kalmar University, Barlastgatan 11 Kalmar SE-391 82, Sweden;
Top