Volume 14, Number 12—December 2008
Letter
Human Case of Bartonella alsatica Lymphadenitis
To the Editor: Lymph node enlargement is a common medical problem that is usually caused by bacterial, viral, fungal, or protozoal agents (1). Malignancies or lymphoproliferative diseases are often found, especially in elderly patients (1). Bartonella henselae, the main causative agent of cat-scratch disease (CSD), appears to be the most common organism responsible for lymphadenopathy in adults and children (1). CSD has also been rarely associated with B. quintana (2). Recently, the epidemiology of B. quintana as an emerging source of human infection has changed because it has been isolated from the dental pulp of a domestic cat (3). Feral cats have also been found to be infected by B. quintana (4). We report a human case of B. alsatica lymphadenopathy.
A 79-year-old woman came to a hospital in Agen, France, in February 2008 with a large painless axillary mass that she had noticed 10 days earlier. She reported that ≈1 month earlier she was scratched on her finger while butchering a wild rabbit. On examination, she did not have any other specific findings. Blood cell counts and levels of liver enzymes were normal. A large necrotic lymph node was surgically removed the next day. Her condition was treated with doxycycline (200 mg) for 3 weeks.
Our laboratory received a fragment of the lymph node of the patient and a portion of the rabbit that had been cooked, boiled as a terrine, and stored in a freezer at –20°C in the home of the patient. DNA was extracted from these specimens by using a QIAamp Tissue Kit (QIAGEN, Hilden, Germany). The DNA was used as a template in 3 described PCRs specific for a portion of the B. alsatica 16S–23S intergenic spacer (ITS) region, ftsZ gene, and 16S rDNA (5). All results for the lymph node were positive for B. alsatica, and amplification products of the expected size were obtained from this extract. Sequences obtained shared 100% similarity with the corresponding 16S rDNA, ITS region, and ftsZ gene fragment of B. alsatica. However, the terrine specimen was negative for 16S rDNA, the ITS region, and the ftsZ gene. All negative controls showed typical results. B. alsatica have not been tested or found in our laboratory for several years.
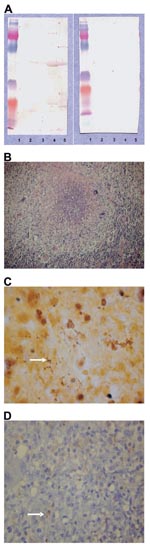
B. quintana subsp. Oklahoma, B. henselae subsp. Houston (ATCC 49882), B. vinsonii subsp. berkhoffi (URBVAIE25), B. vinsonii subsp. arupensis (ATCC 700727), and B. alsatica (CIP 105477 T) strains were used for immunofluorescence and Western blotting assays (5). A serum sample taken at admission was negative for B. alsatica by immunofluorescence assay. This result was accepted because serologic results may be negative during the onset of the disease (6). Western blotting with Bartonella spp. antigens (5) was positive for B. alsatica and after adsorption, only B. alsatica antigens retained all antibodies (Appendix Figure, panel A).
Formalin-fixed, paraffin-embedded tissue specimens (3-μm thick) were stained with hematoxylin and eosin. Microscopic examination showed that the normal architecture of the lymph node was destroyed. Histologic changes were dominated by large irregular stellate or round granulomas with central neutrophil-rich necrosis (Appendix Figure, panel B). Granulomas were composed mainly of macrophages, whereas neutrophils in the necrotic areas were fragmented. These granulomas with abscess formation were similar to those described in CSD. Warthin-Starry staining showed bacteria in the necrotic center of the granulomas (Appendix Figure, panel C).
Immunohistologic staining was used to demonstrate B. alsatica in the lymph node. Immunohistochemical analysis was performed by using a monoclonal antibody against B. alsatica with an immunoperoxidase kit previously described (7). Briefly, after deparaffinization, the tissue section was incubated with polyclonal-specific antibody to B. alsatica (8) diluted 1:1,000 in phosphate-buffered saline. Immunodetection was performed with biotinylated immunoglobulins, peroxidase-labeled streptavidin (HistoStain Plus Kit; Zymed, Montrouge, France), and amino-ethyl-carbazole as substrate. Slides were counterstained with Mayer hematoxylin for 10 min. Location of bacteria was superimposable on that in the Warthin-Starry–stained specimens, and clusters of microorganisms were seen in the inflammatory areas (Appendix Figure, panel D).
We report lymphadenitis caused by B. alsatica. Our finding was confirmed by molecular, serologic, and staining methods. Bartonella spp. are zoonotic agents that infect erythrocytes of mammals in which they cause chronic bacteremia (9). B. alsatica was first identified in 1999 in Alsace, France, as an agent of bacteremia in healthy wild rabbits (10). However, in 2006, interest in B. alsatica increased when it was considered to be a human pathogen because it caused blood-culture–negative endocarditis in a patient who had contacts with rabbits (5). The present case confirms that B. alsatica could be a human pathogen, especially in persons who live in contact with rabbits and should be considered a cause of lymphadenopathy.
References
- Rolain JM, Lepidi H, Zanaret M, Triglia JM, Michel G, Thomas PA, Lymph node biopsy specimens and diagnosis of cat-scratch disease. Emerg Infect Dis. 2006;12:1338–44.PubMedGoogle Scholar
- Raoult D, Drancourt M, Carta A, Gastaut JA. Bartonella (Rochalimaea) quintana isolation in patient with chronic adenopathy, lymphopenia, and a cat. Lancet. 1994;343:977. DOIPubMedGoogle Scholar
- La VD, Tran-Hung L, Aboudharam G, Raoult D, Drancourt M. Bartonella quintana in domestic cat. Emerg Infect Dis. 2005;11:1287–9.PubMedGoogle Scholar
- Breitschwerdt EB, Maggi RG, Sigmon B, Nicholson WL. Isolation of Bartonella quintana from a woman and a cat following putative bite transmission. J Clin Microbiol. 2007;45:270–2. DOIPubMedGoogle Scholar
- Raoult D, Roblot F, Rolain JM, Besnier JM, Loulergue J, Bastides F, First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol. 2006;44:278–9. DOIPubMedGoogle Scholar
- Maurin M, Rolain JM, Raoult D. Comparison of in-house and commercial slides for detection of immunoglobulins G and M by immunofluorescence against Bartonella henselae and Bartonella quintana. Clin Diagn Lab Immunol. 2002;9:1004–9. DOIPubMedGoogle Scholar
- Lepidi H, Fournier PE, Raoult D. Quantitative analysis of valvular lesions during Bartonella endocarditis. Am J Clin Pathol. 2000;114:880–9. DOIPubMedGoogle Scholar
- Bonhomme CJ, Nappez C, Raoult D. Microarray for serotyping of Bartonella species. BMC Microbiol. 2007;7:59. DOIPubMedGoogle Scholar
- Breitschwerdt EB, Kordick D. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–38. DOIPubMedGoogle Scholar
- Heller R, Kubina M, Mariet P, Riegel P, Delacour G, Dehio C, Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int J Syst Bacteriol. 1999;49:283–8.PubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 14, Number 12—December 2008
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Didier Raoult, Unité des Rickettsies, Centre National de la Recherche Scientifique–Institut de Recherche pour le Développement, Unité Mixte de Recherche 6236, Faculté de Médecine, Université de la Méditerranée, 27 Bd Jean Moulin, 13385 Marseille CEDEX 05, France
Top