Volume 15, Number 4—April 2009
Dispatch
Enterovirus 71 Maternal Antibodies in Infants, Taiwan
Abstract
Enterovirus 71 (EV71) causes life-threatening disease outbreaks in young children in Asia. This cohort study was conducted to understand the dynamics of maternal EV71 antibodies in Taiwanese young infants. Approximately 50% of neonates had detectable EV71 neutralizing antibodies, which declined to almost undetectable levels by 6 months of age.
Transplacental maternal antibodies protect young infants from infectious diseases. On the other hand, maternal antibodies in young infants may impede vaccine effectiveness and confound interpretation of vaccine-induced immune responses. Thus, a need exists to understand the dynamics of pathogen-specific maternal antibodies in young infants (1–3).
Enterovirus 71 (EV71) was first isolated in California, USA, in 1969. Since then, EV71 has been isolated globally and causes life-threatening outbreaks in young children in Asia (4–10). National surveillance data and epidemiologic studies show that infants have an increased risk of severe EV71 infections in Taiwan (6–11). Consequently, vaccine development for EV71 in Taiwan should target infants. This cohort study was conducted to understand the dynamics of EV71-specific maternal antibodies in young infants in Taiwan.
Seropositive rates of EV71 neutralizing antibodies in preschool children have been found to be higher in rural areas than in urban areas in Taiwan (11). We chose Chang Gung Memorial Hospital (CGMH) as a study site because it has large obstetric and pediatric populations and serves residents from rural and urban areas in northern Taiwan (7). Pregnant women having prenatal examinations at CGMH were invited to participate in the study. Serum samples were obtained from participating pregnant women and their children to measure EV71 neutralizing antibody titers immediately before delivery for pregnant women; at birth for neonates (cord blood); and at 6, 12, 24, 36, and 48 months of age for infants. Institutional review board approvals were obtained from CGMH and from the National Health Research Institutes, according to the Helsinki Declaration. Informed consent was obtained from all mothers of participating infants. This report addresses the dynamics of EV71 maternal antibodies in young infants by 6 months of age.
Laboratory methods for measuring EV71 serum neutralizing antibody titers followed standard protocols (7) and used a local strain (TW/E59/2002 [B4 genotype]) and rhabdomyosarcoma cells. Serial serum samples obtained from each pregnant woman and her infant were tested in the same run to reduce assay variations. The starting dilution was 1:8, and the cutoff level for seropositivity was 8. Undetectable titer was assigned a level of 2 for calculation of geometric mean titer (GMT). For determining serostatus (positive or negative), serum samples were tested only at 1:8.
Under the assumption that levels of maternal antibodies decline exponentially and constantly, this study used paired serum samples collected at birth and at 6 months of age to estimate the biological half-life that represents an overall half-life and that is crucial for interpreting antibody responses in young infants. Longitudinal and cross-sectional methods of data analysis were used to estimate the biological half-life of pathogen-specific maternal antibodies (1).
Obtaining monthly serum samples from young infants to measure seroprevalence of maternal EV71-specific antibodies is unrealistic. Alternatively, the seroprevalence can be predicted mathematically. As has been shown in other viral pathogens, maternal antibody titers are assumed to follow a normal distribution after natural logarithm transformation and to experience a constant exponential decay over time after an infant’s birth (1,12). If we assume normal distribution, 4 parameters (initial GMT at age i, SD of the distribution of antibody titers, decay rates of antibody titers, and cut-off for seropositivity) are crucial for estimating the seroprevalence in different ages (12).
Neutralization antibody titers were log-transformed to calculate the GMT and 95% confidence intervals. Statistical association between 2 nominal or ordinary variables was tested by using the χ2 test, McNemar test, Fisher exact test, or Mantel-Haenszel χ2 test, as appropriate. All statistical analyses were performed using Microsoft Excel (Microsoft, Redmond, WA, USA) or SAS software (SAS Institute, Cary, NC, USA).
Serum samples from 459 pregnant women and their neonates were obtained from June 2006 to June 2008 and tested for EV71 neutralizing antibody serostatus. Seropositive rates of EV71 neutralizing antibodies in these pregnant women and their neonates were 63% and 51%, respectively. Seroprevalence in mothers was strongly associated with seroprevalence in their neonates, and neonates born to seronegative mothers were all seronegative (p<0.01, by McNemar test). In addition, the EV71 antibody titers in seropositive neonates were highly correlated with the EV7 antibody titers in their mothers (R = 0.84, p<0.01) (Figure 1).
From June 2006 through June 2008, a total of 309 neonates completed follow-up and blood collection at 6 months of age. EV71 neutralizing antibody titers in the serial serum samples from these 309 families were quantified. The seropositive rates of EV71 neutralizing antibodies in the family cohorts were 65% in the mothers, 50% in the neonates, and 1% in the 6-month-old infants, respectively (Table 1). Only 4 infants 6 months of age had detectable EV71 antibody titers; 1 showed seroconversion on the basis of antibody titers measured at birth (antibody titer <8) and at 6 months of age (antibody titer 512). This seroconverted infant did not develop any enterovirus-related symptoms (e.g., hand, foot, and mouth disease or herpangina). Of the 154 seropositive neonates, 3 remained seropositive and 151 became seronegative at 6 months of age (Table 2). In the 3 seropositive 6-month-old infants, the biological half-life of EV71 neutralizing antibodies was calculated as 39 and 42 days by using cross-sectional and longitudinal analyses, respectively. In the 151 seronegative 6-month-old infants, the biological half-life of EV71 neutralizing antibody was calculated as 53 and 60 days by using cross-sectional and longitudinal analysis, respectively (Table 2).
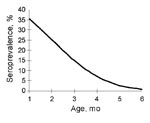
Seroprevalence rates were predicted by making the assumptions that the seropositive rate of EV71 neutralizing antibodies at birth is 50%, that the GMT of these seropositive neonates is 21.8 (SD 0.91), and that the half-life of maternal EV71 neutralizing antibodies is 42 days (1.4 months). Seroprevalence rates of EV71 neutralizing antibodies during each of the first 6 months of age were predicted to be 35%, 25%, 14%, 7%, 3%, and 1%, respectively (Figure 2). The predicted rate at 6 months of age was consistent with the observed seroprevalence at 6 months of age in our study population.
We found that ≈50–60% of pregnant women had serum EV71 neutralizing antibodies. These maternal EV71 neutralizing antibodies declined to undetectable levels in 99% of the 6-month-old infants. Two cross-sectional studies found similar seropositive rates in 30–49-year-old adults and in 6–11-month-old infants before the 1998 epidemic in Taiwan (10,11). In Singapore, a cross-sectional study found that 44% of 70 neonates had EV71 neutralizing antibodies in cord blood samples, but none of 52 infants 1–11 months old was seropositive (13).
Studies estimating the biologic half-life of EV71 maternal antibodies appear to be new. Theoretically, longitudinal analysis is more reliable and has narrower confidence intervals than cross-sectional analysis (1). Although only 3 infants had detectable antibody titers at 6 months of age, our study estimates the biological half-life of EV71 maternal antibodies to be 42 days, similar to the half-lives of antibodies to other pathogens calculated by using longitudinal analysis (1; M.-S. Lee, unpub. data).
Our prospective serologic study in northern Taiwan showed no seroconversion in young infants during 2007 and only 1 seroconversion during the first half of 2008. Based on the national enterovirus surveillance system, EV71 isolations were very low in 2006 and 2007 (14,15), findings consistent with our study.
A national program for developing EV71 vaccines was initiated in Taiwan in 2007. To improve vaccine development, the target population (those at high risk) needs to be identified. Several studies have shown that infants 6–11 months of age in Taiwan have the highest risk for severe EV71 infection and for death caused by this infection (8–10). Our serologic study found that 99% of 6-month-old infants have undetectable maternal EV71 neutralizing antibodies. Consequently, EV71 vaccines being developed in Taiwan should target infants <6 months of age.
Ms Luo is an epidemiologist in the Vaccine Research and Development Center, National Health Research Institutes. Her research interests include the epidemiology of infectious diseases and clinical trials.
Acknowledgments
We thank the referring obstetrician and mothers of participating infants, Mei-Liang Huang for stimulating discussion, Pele Chong and Mark Swofford for manuscript review, and Taiwan Centers for Disease Control for providing virus strains.
The study was funded by the National Health Research Institutes, Taiwan.
References
- Lee MS, Mendelman PM, Sangli C, Cho I, Mathie SL, August MJ. Half-life of human parainfluenza virus type 3 (hPIV3) maternal antibody and cumulative proportion of hPIV3 infection in young infants. J Infect Dis. 2001;183:1281–4. DOIPubMedGoogle Scholar
- Lee MS, Greenberg DP, Yeh SH, Yogev R, Reisinger KS, Ward JI, Antibody responses to bovine parainfluenza virus type 3 (PIV3) vaccination and human PIV3 infection in young infants. J Infect Dis. 2001;184:909–13. DOIPubMedGoogle Scholar
- Greenberg DP, Walker RE, Lee MS, Reisinger KS, Ward JI, Yogev R, A bovine parainfluenza virus type 3 vaccine is safe and immunogenic in early infancy. J Infect Dis. 2005;191:1116–22. DOIPubMedGoogle Scholar
- Bible JM, Pantelidis P, Chan PK, Tong CY. Genetic evolution of enterovirus 71: epidemiological and pathological implications. Rev Med Virol. 2007;17:371–9. DOIPubMedGoogle Scholar
- World Health Organization. Outbreak news. Enterovirus, China. Wkly Epidemiol Rec. 2008;83:169–70.PubMedGoogle Scholar
- Lin TY, Twu SJ, Ho MS, Chang LY, Lee CY. Enterovirus 71 outbreaks, Taiwan: occurrence and recognition. Emerg Infect Dis. 2003;9:291–3.PubMedGoogle Scholar
- Chang LY, Tsao KC, Hsia SH, Shih SR, Huang CG, Chan WK, Transmission and clinical features of enterovirus 71 infections in household contacts in Taiwan. JAMA. 2004;291:222–7. DOIPubMedGoogle Scholar
- Chang LY, Huang LM, Gau SS, Wu YY, Hsia SH, Fan TY, Neurodevelopment and cognition in children after enterovirus 71 infection. N Engl J Med. 2007;356:1226–34. DOIPubMedGoogle Scholar
- Chen SC, Chang HL, Yan TR, Cheng YT, Chen KT. An eight-year study of epidemiologic features of enterovirus 71 infection in Taiwan. Am J Trop Med Hyg. 2007;77:188–91.PubMedGoogle Scholar
- Lu CY, Lee CY, Kao CL, Shao WY, Lee PI, Twu SJ, Incidence and case-fatality rates resulting from the 1998 enterovirus 71 outbreak in Taiwan. J Med Virol. 2002;67:217–23. DOIPubMedGoogle Scholar
- Chang LY, King CC, Hsu KH, Ning HC, Tsao KC, Li CC, Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics. 2002;109:e88. DOIPubMedGoogle Scholar
- Lee MS, Nokes DJ. Predicting and comparing long-term measles antibody profiles of different immunization policies. Bull World Health Organ. 2001;79:615–24.PubMedGoogle Scholar
- Ooi EE, Phoon MC, Ishak B, Chan SH. Seroepidemiology of human enterovirus 71, Singapore. Emerg Infect Dis. 2002;8:995–7.PubMedGoogle Scholar
- Huang YP, Lin TL, Kuo CY, Lin MW, Yao CY, Liao HW, The circulation of subgenogroups B5 and C5 of enterovirus 71 in Taiwan from 2006 to 2007. Virus Res. 2008;137:206–12. DOIPubMedGoogle Scholar
- Taiwan Centers for Disease Control. Sentinel surveillance weekly report [cited 2008 Nov 7]. Available from http://www.cdc.gov.tw
Figures
Tables
Cite This ArticleTable of Contents – Volume 15, Number 4—April 2009
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Min-Shi Lee, Vaccine Research and Development Center, National Health Research Institutes, R1-7F, 35 Keyan Rd, Zhunan, Miaoli County 350, Taiwan
Top