Volume 15, Number 8—August 2009
Research
Dengue 1 Virus and Dengue Hemorrhagic Fever, French Polynesia, 2001
Abstract
An epidemic of dengue 1 virus (DENV-1) occurred in French Polynesia in 2001, 4 years after a DENV-2 epidemic that ended in 1997. Surveillance data from hospitalized case-patients showed that case-patients with dengue hemorrhagic fever (DHF) exhibited a bimodal age distribution with 1 peak among infants 6–10 months of age and a second peak at 4–11 years of age. The relative risk of DHF developing in children born before rather than after the DENV-2 epidemic was 186 (95% confidence interval 26–1,324). Among children born toward the end of the DENV-2 epidemic, a strong temporal association was found between the month of birth and the risk of being hospitalized for DHF. This study documents epidemic pathogenicity associated with the sequence of DENV-2 infection followed by DENV-1 infection.
Four dengue virus serotypes (DENV-1 to DENV-4) are transmitted to humans. In some infected persons a milder form, dengue fever (DF), may develop, whereas in a smaller proportion, the severe disease, dengue hemorrhagic fever (DHF), develops, which is characterized by an excessive capillary permeability that may lead to shock (dengue shock syndrome [DSS]) and death.
Island populations provide unique opportunities to study the epidemiology and pathogenesis of introduced pathogens. Of note have been the dengue epidemics that have succeeded the introduction of DENV-1 into Cuba and its transmission during 1977–1978 to nearly 45% of the population. An Asian genotype of DENV-2 was introduced into Cuba in 1981 and caused a major epidemic of DF and DHF across the population of a wide range of ages, beginning with those 3 years of age (1). In 1997, an Asian DENV-2 virus was again introduced into the city of Santiago de Cuba and DF and DHF were again observed, this time in persons >20 years of age (2). In 2001, DENV-3 appeared in Havana and its environs, and again DF and DHF cases were observed only in adults (3). The epidemiologic situation in French Polynesia resembles that of Cuba because, over the past 50 years, at least 10 different DENVs have resulted in epidemics (4). Each epidemic was associated with the recovery of only 1 serotype and generally was succeeded during interepidemic periods by low transmission of that same DENV.
A recent DENV-1 epidemic has provided an opportunity to relate the occurrence of DHF to a history of exposure of the population to another dengue serotype. Four years after a large DENV-2 epidemic that began in 1996 and affected all of French Polynesia, ending in 1997, in January 2001 DENV-1 virus was identified on Bora Bora Island. Over the next 10 months, this epidemic spread to all of French Polynesia. The number of dengue-like syndromes diagnosed by general practitioners in the Society Islands was estimated to be 33,000 (16% of the overall population) (5). We report an analysis of the distribution of hospitalized case-patients by date of birth and our conclusion that severe disease in 2001 resulted from infections in the sequence of DENV-2 followed by DENV-1.
Geographic Background
French Polynesia (235,000 inhabitants in 2001), located 4,400 km southeast of Hawaii, includes 4 archipelagos. The most highly populated, Society Archipelago (202,000 inhabitants), includes 7 inhabited islands including Tahiti and Bora Bora. The 3 other archipelagoes, considered as outer islands, are the Marquesas, Tuamotu, and Australes Islands (Figure 1).
History of Dengue Activity in French Polynesia
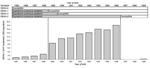
Ten outbreaks with DENVs isolated have been documented since 1944 (Table). Except for a DENV-2 epidemic in 1971 and a DENV-3 epidemic in 1990, the severity of disease in other epidemics was mild (4). In August 1996, cases of DF were reported to the Directorate of Health for French Polynesia. These were shown to be caused by DENV-2. The scope and duration of the epidemic are illustrated in Figure 2.
Surveillance
In 2001, surveillance of DF cases in hospitalized patients was established in all 7 hospitals in French Polynesia, 4 of them on Tahiti Island, and the 3 others in Raiatea (close to Bora Bora), Moorea, and Hiva Oa. Hospital-based physicians were asked to complete a questionnaire that included demographic, clinical, and biologic information on each patient admitted with a diagnosis of dengue regardless of severity. Completed questionnaires were sent to the epidemiologic unit of the Directorate of Health at the time the patient was discharged from the hospital.
Case Definition
Hospitalized case-patients were classified as having DF, DHF, or DSS, according to World Health Organization guidelines (6). Because the tourniquet test was rarely performed, case-patients with a history of fever, thrombocytopenia, and evidence of plasma leakage but without spontaneous bleeding phenomena were classified as having DHF grade I.
Laboratory Studies
Confirmation of DENV infection was obtained from case-patients by reverse transcription–PCR or virus isolation at an early stage of the disease or by immunoglobulin (Ig) M and IgG detection during hospitalization. Second serum specimens were rarely obtained after hospitalization. Laboratory analyses to detect antibodies against DENV by using IgM capture and IgG ELISAs were performed as previously described (7). Isolation and identification of the virus were performed at the Laboratoire de Recherche en Virologie Médicale, Institut Louis Malardé, Papeete, Tahiti. Results are reported elsewhere (8).
Analysis
DHF incidence rates were computed by reported age and, for some analyses, by year and month of birth. A more in-depth analysis by month of birth was performed on hospitalized children with DENV-1 infections born in 1996 or 1997. The hypothesis being tested was that, as the 1996–7 DENV-2 epidemic waned, infection rates with this serotype will decline and, during the 2001 DENV-1 epidemic, the disappearance of those immune to DENV-2 will correlate with a reduction in severe cases. As a proxy of the monthly evolution of the risk of being DENV-2 infected from August 1996 through June 1997, we used the monthly cumulative percentage of patients still not infected among the 2,035 DENV-2 confirmed cases during this period (Figure 2).
Statistical Analysis
All reported p values are 2-sided. Statistical analyses were performed with Epi-Info V6.4d (Centers for Disease Control and Prevention, Atlanta, GA, USA).
During the 2001 epidemic, 1,379 persons were hospitalized with a diagnosis of possible DENV infection (9/1,000 population). Among these, 256 were confirmed by virus isolation or reverse transcription–PCR and 420 additional cases by serologic analysis. All viruses and RNA recovered were DENV-1. Among hospitalized case-patients, 746 (54%) were classified as DF, 157 (11%) as DHF grade I, 198 (14%) as DHF grade II, and 278 (20%) as DSS (DHF grade III or IV). The overall incidence rate of DHF was 2.7 per 1,000 population. Eight fatal cases were reported; 7 were DSS cases in patients 5–12 years of age.
DHF Case-Patients by Age
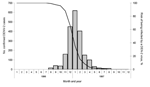
Ninety-seven percent of DHF cases occurred among persons <20 years of age. The age distribution of hospitalized and of DHF case-patients exhibited a bimodal distribution with 1 peak in infants, followed by a 3-year gap in severe cases, an increase in severe cases among 4 year-olds and a steep increase in 5–11 year age group (Figure 3, panel A). Among children 5–11 years of age, a linear declining trend was observed with the DSS rate falling from 11.6/1,000 to 3.1/1,000 (p<0.001, by χ2 test for trend). Among the 63 hospitalized children <2 years of age, 27 had DHF. Seventy percent of hospitalized case-patients and, among hospitalized patients, 88% of DHF case-patients were 4–11 months of age; the incidence peaked at 8 months of age (Figure 3, panel B). Among those 12–24 months of age, only 2 DHF case-patients were observed.
DHF Case-Patients by Date of Birth
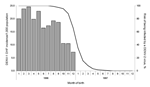
DHF attack rates fell sharply among children born earlier than 1989 (the year of a previous DENV-1 epidemic) (Figure 4). In 2001, only 1 child born during 1997–1999 (after the DENV-2 epidemic) had DHF. The relative risk for DHF to develop in a child that was born in 1990–1996 (499/34,000) versus being born in1997–1999 (1/12,900) was 186 (95% confidence interval 26–1,324). Among children born during the second half of 1996, the DHF incidence rates decreased by month of birth and fell to 0 among those born in January 1997. This decrease was parallel to the risk of being subsequently infected by DENV-2 during 1996, with a 2-month delay between birth and the risk for infection (Figure 5).
Although the evidence linking DHF/DSS with a second dengue infection is substantial, the proposed pathogenetic mechanisms of DHF/DSS that explain why dengue infections occur in the presence of circulating antibodies are still controversial (9). Two theories, not mutually exclusive, are frequently cited. The most commonly accepted is the antibody-dependent enhancement hypothesis in which dengue disease severity is modified by enhanced infections in monocytes and macrophages that result from infection by immune complexes formed by virus and antibodies raised from prior infection with a heterologous DENV or passively acquired at birth (10). The second theory is that DENVs differ in virulence or fitness. DHF risk is found most notably during secondary DENV-2 and DENV-3 infections, whereas some genotypes, notably the American genotype DENV-2, have reduced severity potential compared with Asian genotype viruses (11–13). Both hypotheses are consistent with an increased circulation of DENVs in the world with a possible selection of the fittest strains and an increased probability of secondary infections.
We describe the bimodal nature of age distribution in DHF in French Polynesia. This phenomenon was described in Thailand in 1962–1964 (14) and in Cuba in 1981 (1). This feature, unique among human infectious diseases, supports the hypothesis that DHF in infants is explained by enhanced dengue infections resulting from passively acquired maternal dengue antibodies (15). Among hospitalized infants with DF/DHF observed in French Polynesia in 2001, the age distribution of DHF cases was similar to age distributions of case-patients hospitalized in 4 Southeast Asian countries where all 4 dengue serotypes are highly endemic. In French Polynesia, as in Thailand, the incidence of DHF was 15/1,000 in 8-month-old infants (16). The age distribution of infant case-patients has been attributed to the catabolism of maternal antibodies following parturition (17). This degradation of antibodies creates 3 successive phases of risk for dengue infection or disease in the infant: 1) a protection phase between birth and the age of 5 months, 2) a period that follows in which the antibodies no longer neutralize but rather facilitate severe infections, and finally, 3) the complete disappearance of these antibodies when the child is ≈11 months of age, leaving the child open to a so-called normal infection. These data and interpretations were confirmed in recent studies on infant DHF in Vietnam (18,19).
In this study, the incidence of DHF in children >1 year of age, when arranged by date of birth, demonstrated the major risk factor was to have been born before the DEN-2 epidemic. In addition, children born before 1989, and exposed to an earlier DENV-1 epidemic, had a reduced risk of being hospitalized during the 2001 epidemic and were apparently protected by having acquired DENV-1 immunity. An even more striking result is the decreasing incidence rate of DHF by month of birth among children born at the interface between high and low transmission of DENV-2 epidemic. This association was even stronger if a 2-month delay between birth and risk for infection is considered. Explanations include the possibility that very young infants may be shielded from mosquito bites or that maternal antibodies are highly protective during this interval (20).
Another notable finding in this study is that the incidence of DSS steadily declined among children 5–11 years of age. This finding of a decline resembles an observation made concerning the 1981 DHF epidemic in Cuba: that DHF/DSS age-specific incidence rates were inversely related to age (21). This finding is also consistent with results of studies about normal human endothelial function. Gamble et al. found a marked decline in filtration capacity (a factor describing vascular permeability) in the first 2 decades of life and hypothesized that the incidence of DSS might be age dependent (22). Bethell et al. demonstrated that children with DHF are more likely than adults to progress to the DSS state because their baseline microvascular permeability is higher (23).
Based upon envelope glycoprotein sequence analyses, the 2001 Tahitian DENV-1 strain was found to be similar to strains isolated in Indonesia in 1988 and was likely of Southeast Asian origin, in contrast to the 1988–1989 Tahitian DENV-1 strain, which was found to be of Asian-American origin (8,24). A complete nucleotide sequence analysis confirmed that the same DENV-1 virus spread from French Polynesia to Hawaii and Easter Island (25). It is noteworthy that in those sites where no other DENVs have circulated for many years, DHF cases were not observed during these DENV-1 outbreaks (26,27). This finding is in agreement with our observation that severe disease did not develop in any children, 1–3 years of age, who were born after the DENV-2 epidemic in French Polynesia, although the youngest children are at highest risk for dengue vascular permeability syndrome (21). This comprises supportive evidence that the DENV-1 circulating in Tahiti in 2001 was not inherently virulent.
Earlier epidemic occurrences of DHF have been primarily associated with DENV-2 or DENV-3. The large Cuban epidemic of 1981 was DENV-2, which followed DENV-1 (1). Notably, in the 1980 dengue outbreak in Rayong, Thailand, no severe disease was observed in any infection sequence that ended with DENV-1, including DENV-2, followed by DENV-1 (28). Although, in other years and places, secondary DENV-1 infections have resulted in DHF, we found epidemic DHF that accompanied sequential infection in the specific sequence of DENV-2 followed by DENV-1 (29,30). This outbreak resulted in a DHF/DSS incidence rate of 2.7/1,000 population, similar to the 3.2/1,000 rate observed during a large epidemic in Thailand in 1987 in which secondary DENV-2 and DENV-3 virus infections predominated (6). The reasons for changes in pathogenicity of secondary DENV-1 infections require further careful study.
Dr Hubert is a medical epidemiologist in the Institut de Veille Sanitaire in France. His interests include surveillance and outbreak investigations in the field of infectious diseases. At the time of this investigation, he was responsible for communicable diseases programs in the Directorate of Health of French Polynesia.
Dr Halstead is adjunct professor in the Department of Preventive Medicine and Biometrics, Uniformed Services University of the Health Sciences, Bethesda, Maryland. His research interests include the epidemiology, pathogenesis, and immunology of flavivirus infections and vaccines for DF and Japanese encephalitis.
Acknowledgment
We gratefully acknowledge the physicians who participated in the surveillance of hospitalized case-patients in French Polynesia.
References
- Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–84 .PubMedGoogle Scholar
- Guzman MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vazques S, Epidemiologic studies on Dengue in Santiago de Cuba, 1997. Am J Epidemiol. 2000;152:793–9 and. DOIPubMedGoogle Scholar
- Rodriguez-Roche R, Alvarez M, Holmes EC, Bernardo L, Kouri G, Gould EA, Dengue virus type 3, Cuba, 2000–2002. Emerg Infect Dis. 2005;11:773–4 . DOIPubMedGoogle Scholar
- Chungue E, Deparis X, Murgue B. Dengue in French Polynesia: major features, surveillance, molecular epidemiology and current situation. Pac Health Dialog. 1998;5:154–62.
- Hubert B. Type 1 dengue fever epidemic in French Polynesia—2001 [monograph on the Internet] [cited 2009 Feb 20]. Available from http://www.spc.int/phs/PPHSN/Outbreak/reports/Dengue_report2001-FrenchPolynesia.pdf
- World Health Organization. Dengue haemorrhagic fever. Diagnosis, treatment, prevention and control. Geneva: The Organization; 1997.
- Chungue E, Boutin JP, Roux J. Significance of IgM titration by an immunoenzyme technique for the serodiagnosis and epidemiological surveillance of dengue in French Polynesia [in French]. Res Virol. 1989;140:229–40 and. DOIPubMedGoogle Scholar
- Laille M, Roche C. Comparison of dengue-1 virus envelope glycoprotein gene sequences from French Polynesia. Am J Trop Med Hyg. 2004;71:478–84 .PubMedGoogle Scholar
- Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–67 and. DOIPubMedGoogle Scholar
- Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–81 and. DOIPubMedGoogle Scholar
- Vaughn DW. Invited commentary: dengue lessons from Cuba. Am J Epidemiol. 2000;152:800–3 and. DOIPubMedGoogle Scholar
- Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96 .PubMedGoogle Scholar
- Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–51 and. DOIPubMedGoogle Scholar
- Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969;18:954–71 .PubMedGoogle Scholar
- Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis. 2002;8:1474–9 . DOIPubMedGoogle Scholar
- Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411–9 .PubMedGoogle Scholar
- Watanaveeradej V, Endy TP, Samakoses R, Kerdpanich A, Simasathien S, Polprasert N, Transplacentally transferred maternal–infant antibodies to dengue virus. Am J Trop Med Hyg. 2003;69:123–8 .PubMedGoogle Scholar
- Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, Dung NT, Dengue in Vietnamese infants–results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198:516–24 and. DOIPubMedGoogle Scholar
- Simmons CP, Chau TN, Thuy TT, Tuan NM, Hoang DM, Thien NT, Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis. 2007;196:416–24 and. DOIPubMedGoogle Scholar
- Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–46 and. DOIPubMedGoogle Scholar
- Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis. 2002;6:118–24 and. DOIPubMedGoogle Scholar
- Gamble J, Bethell D, Day NP, Loc PP, Phu NH, Gartside IB, Age-related changes in microvascular permeability: a significant factor in the susceptibility of children to shock? Clin Sci (Lond). 2000;98:211–6 and. DOIPubMedGoogle Scholar
- Bethell DB, Gamble J, Pham PL, Nguyen MD, Tran TH, Ha TH, Noninvasive measurement of microvascular leakage in patients with dengue hemorrhagic fever. Clin Infect Dis. 2001;32:243–53 and. DOIPubMedGoogle Scholar
- Imrie A, Zhao Z, Bennett SN, Kitsutani P, Laille M, Effler P. Molecular epidemiology of dengue in the Pacific: introduction of two distinct strains of the dengue type-1 virus into Hawaii. Ann Trop Med Parasitol. 2006;100:327–36 and. DOIPubMedGoogle Scholar
- Caceres C, Yung V, Araya P, Tognarelli J, Villagra E, Vera L, Complete nucleotide sequence analysis of a dengue-1 virus isolated on Easter Island, Chile. Arch Virol. 2008;153:1967–70 and. DOIPubMedGoogle Scholar
- Perret C, Abarca K, Ovalle J, Ferrer P, Godoy P, Olea A, Dengue-1 virus isolation during first dengue fever outbreak on Easter Island, Chile. Emerg Infect Dis. 2003;9:1465–7 . DOIPubMedGoogle Scholar
- Effler PV, Pang L, Kitsutani P, Vorndam V, Nakata M, Ayers T, Dengue fever, Hawaii, 2001–2002. Emerg Infect Dis. 2005;11:742–9 . DOIPubMedGoogle Scholar
- Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120:653–69 .PubMedGoogle Scholar
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9 and. DOIPubMedGoogle Scholar
- Gibbons RV, Kalanarooj S, Jarman R, Nisalak A, Vaughn DW, Endy TP, Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007;77:910–3 .PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 15, Number 8—August 2009
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Bruno Hubert, Cellule Inter-Régionale d’Épidémiologie, BP 86218, 44262 Nantes Cedex 2, France
Top