Volume 16, Number 10—October 2010
Letter
Dictyostelium polycephalum Infection of Human Cornea
To the Editor: Although Dictyostelium spp. are used for studying signal transduction, cytoskeletal functions, endocytosis, and molecular pathogenesis of infectious and other diseases (1), human or animal infections caused by this organism have not been reported. We report a case of keratitis caused by Dictyostelium polycephalum in an immunocompetent person.
A 35-year-old man sought treatment for redness, pain, and watering in the left eye of 11 days’ duration. He had no history of ocular injury or surgery. At the time of his medical visit, he was using ophthalmic solutions of 5% natamycin sulfate, 0.5% moxifloxacin hydrochloride, and 0.3% gentamicin sulfate, each instilled every hour, and 1% atropine sulfate, 3×/d.
The vision in his right eye and results of a clinical examination were within normal limits. His left eye visual acuity was expressed as the ability to count fingers at 1 m. The eyelids were edematous and the conjunctivae were congested. The cornea showed a large central epithelial defect with underlying stromal infiltrate and Descemet folds. The surrounding cornea had a mild cellular reaction. The anterior chamber was deep, and the pupil was round, regular, and dilated. Iris and lens details could not be distinguished because of corneal haze. We obtained corneal scrapings, and the material was subjected to a detailed microbiologic analysis (2).
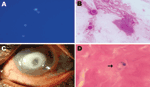
Microscopic examination showed double-walled spherical cysts in potassium hydroxide with calcofluor white stain, Gram stain (Figure, panels A, B), and Giemsa stain. On the basis of this finding, a presumptive diagnosis of Acanthamoeba keratitis was made. The patient was advised to use 0.02% polyhexamethylene biguanide and 0.02% chlorhexidine eye drops every half hour and 1% atropine eye drops 3×/d and was asked to return for a follow-up visit the next day. However, the patient did not return and could not be located. After 48 hours’ of incubation, a nonnutrient agar plate showed growth of double-walled, spherical cysts ≈6–7 µm in diameter that had different morphologic features than those of Acanthamoeba spp. cysts.
To identify the organism, we extracted DNA from the growth on nonnutrient agar and subjected it to PCR specific for Acanthamoeba spp (3); results were negative. The extracted DNA was then subjected to 18S rDNA PCR for free-living amebas as described by Tsvetkova et al. (4). A PCR product ≈800 bp was obtained and subjected to bidirectional sequencing with fluorescent-labeled dideoxy nucleotide terminators by using ABI 3130 XI automated sequencer in accordance with the manufacturer’s instructions (PE Applied Biosystems, Foster City, CA, USA).
The Mega BLAST search program (www.ncbi.nlm.nih.gov/blast/megablast.shtml) of GenBank identified the sequence as D. polycephalum (99% similarity with AM168056). We deposited the sequence of our isolate in GenBank (accession no. GU562439). The organism showed cytotoxicity after in vitro inoculation of a rabbit corneal epithelial cell line.
The patient sought treatment 4 months after his initial visit. The left eye visual acuity was now expressed as the ability to see hand movements near the face. Slit-lamp examination showed lid edema and conjunctival congestion. The cornea showed a ring-shaped infiltrate, central thinning, surrounding corneal edema, and pigments on the endothelium (Figure, panel C); these findings were identical to the clinical picture of Acanthamoeba keratitis. Repeat corneal scrapings showed organisms of same morphologic features seen on the first visit by microscopy and culture. Organisms were reidentified as D. polycephalum by sequencing.
Because we were not aware of any drug treatment recommendations for infection by this organism, and the disease was advanced, surgical treatment was advised. Deep anterior lamellar keratoplasty was performed after 2 days. Histopathologic examination of the corneal button showed spherical cysts in mid stroma and inflammatory infiltrates (Figure, panel D). At the last follow-up (3 months after surgery), the corneal graft was clear with no evidence of infection.
Members of the genus Dictyostelium (social amebas or cellular slime molds) are divided into 4 high-level taxa with several species on the basis of DNA phylogeny (5). The life cycle of Dictyostelium spp. consists of an ameboid vegetative phase, a cyst phase, and a plantlike fruiting phase (6). D. polycephalum is ancestral and show different characteristics than other species of Dictyostelium (5,7,8). In culture, it grows at a temperature of 34°C–35°C, which is higher than that for other species of Dictyostelium (8). Most myxamoebae aggregate to form sporocarps; however, some may round up in individual cells to form microcysts (8).The D. polycephalum isolated from our patient grew at 36°C on nonnutrient agar with an Escherichia coli overlay. The myxamoebae were seen after 24 hours, and the amebae had transformed into microcysts after 48 hours of incubation. However, on further incubation for 3 weeks at 36°C, no sporocarp formed.
Although we could identify the microorganism, the source of infection is unknown. Because the patient was a manual laborer, he could have become infected with the organism from contaminated water or soil. The clinical picture for keratitis caused by D. polycephalum was indistinguishable from that caused by Acanthamoeba spp. However, careful attention to cyst morphology in clinical samples and culture enabled us to identify this organism.
Acknowledgment
This study was supported by the Hyderabad Eye Research Foundation, Hyderabad, India.
References
- Annesley SJ, Fisher PR. Dictyostelium discoideum—a model for many reasons. Mol Cell Biochem. 2009;329:73–91. DOIPubMedGoogle Scholar
- Choudhuri KK, Sharma S, Garg P, Rao GN. Clinical and microbiological profile of Bacillus keratitis. Cornea. 2000;19:301–6. DOIPubMedGoogle Scholar
- Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001;39:1903–11. DOIPubMedGoogle Scholar
- Tsvetkova N, Schild M, Panaiotov S, Mintcheva RK, Gottstein B, Walochnik J, The identification of free living environmental isolates of amoebae from Bulgaria. Parasitol Res. 2004;92:405–13. DOIPubMedGoogle Scholar
- Schaap P, Winkler T, Nelson M, Curto EA, Elgie B, Hagiwara H, Molecular phylogeny and evolution of morphology in social amoeba. Science. 2006;314:661–3. DOIPubMedGoogle Scholar
- Blaskovics JC, Raper KB. Encystment stages of Dictyostelium. Biol Bull. 1957;113:58–88. DOIGoogle Scholar
- Bonner JT. Migration in Dictyostelium polycephalum. Mycologia. 2006;98:260–4. DOIPubMedGoogle Scholar
- Raper KB. Dictyostelium polycephalum n.sp.: a new cellular slime mold with coremiform fructification. J Gen Microbiol. 1956;14:716–32.PubMedGoogle Scholar
Figure
Cite This Article1Current affiliation: GHR Micro Diagnostics,Hyderabad, India.
Related Links
Table of Contents – Volume 16, Number 10—October 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Ashok Kumar Reddy, GHR Micro Diagnostics, 145 Dwarakapuri Colony, Punjagutta, Hyderabad 500 082, India
Top