Volume 18, Number 4—April 2012
Dispatch
Coccidioides posadasii Infection in Bats, Brazil
Abstract
To analyze the eco-epidemiologic aspects of Histoplasma capsulatum in Brazil, we tested 83 bats for this fungus. Although H. capsulatum was not isolated, Coccidioides posadasii was recovered from Carollia perspicillata bat lungs. Immunologic studies detected coccidioidal antibodies and antigens in Glossophaga soricina and Desmodus rotundus bats.
Studies have demonstrated that bats (order Chiroptera) are reservoirs for many infectious agents, including protozoa, bacteria, viruses, and fungi (1). Several studies confirm that bats have a great effect on human health because they can transmit numerous infectious agents and provide a reservoir for emerging pathogens (1,2). The interaction between these animals and pathogenic fungi is well illustrated by the occurrence of histoplasmosis outbreaks in humans who are exposed to bat droppings in the environment (3,4). In Brazil, histoplasmosis is an endemic disease that occurs mainly in patients with AIDS (5), but Histoplasma capsulatum var. capsulatum has also been isolated from bats captured in urban areas (4).
To analyze the eco-epidemiologic aspects of H. capsulatum in northeast Brazil, we captured bats from urban and rural areas of Ceará State. However, the research revealed the existence of a bat that was naturally infected with Coccidioides posadasii and 2 other chiropterans with coccidioidal immunologic responses. This fungal pathogen can cause coccidioidomycosis, a serious infection in humans and animals. The mycosis is presently considered to be endemic to Northeast Brazil, as evidenced by human autochthonous cases (6–8), positive coccidioidin skin-test results (7), and isolation of the fungus from soil (7,9). We describe the isolation of C. posadasii in bats and discuss the epidemiologic effects of this finding.
From August 2010 to March 2011, a total of 83 bats of 7 species were captured in 6 cities in Ceará State, Northeast Brazil, where patients with histoplasmosis are seen: Ubajara, Itapiúna, Quixadá, Russas, Aracoiaba, and Baturité. The animals were captured during the day (nonhematophagous bats) or night (hematophagous bats) by using nylon mist nets with 36-mm mesh. The study was part of the rabies control surveillance program headed by the Ceará State Health Department and was approved by the ethics committee of the State University of Ceará (process 07381395–8).
Immediately after capture, the bats were euthanized by an overdose of diethyl ether by inhalation, and their spleen, liver, and lungs were analyzed for H. capsulatum isolation. Fragments of each organ were homogenized by maceration in saline supplemented with 200 mg/L chloramphenicol. Aliquots of 100 µL were seeded onto plates containing brain–heart infusion agar, supplemented with 1% glucose, 0.1%
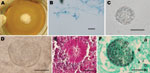
Although none of the samples were positive for H. capsulatum, a colony (Figure) suggestive of Coccidioides spp. (Figure, panel A) was isolated from lung homogenates (incubated at 35°C) from Carollia perspicillata bats. Microscopic analysis showed hyaline septate hyphae and arthroconidia alternating with empty disjunctor cells (Figure, panel B). Lung fragments from the infected bat were then removed from storage and examined by direct microscopy, revealing coccidioidal spherules (Figure, panel C). The suspected Coccicioides colony was evaluated through the in vivo reversion test (9). In brief, 5 mL of 0.9% saline was added to a well-sporulating slant culture (15 days old) that was then gently scraped with a cotton swab. Two mice were injected intraperitoneally with 1 mL of the homogeneous supernatant and then were held under biosafety level 3 conditions for 4 weeks. After this period, the animals were euthanized and their spleen, liver, and lungs were removed. Fragments were examined for coccidioidal spherules by direct microscopy with 10% potassium hydroxide and also cultured on BBL Mycosel Agar (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Additional histopathologic analyses of each organ were performed. Spherules with endospores were found in the lungs of the infected animals (Figure, panel D), and histopathologic analysis supported the identification of Coccidioides spp. (Figure, panels E, F). Fragments of the spleen, liver, and lungs cultured on Mycosel Agar yielded mold colonies that produced typical coccidioidal arthroconidia. An additional test was performed by specific PCR reaction (11) with Coi9–1F (5′-TACGGTGTAATCCCGATACA-3′) and Coi9–1R (5′-GGTCTGAATGATCTGACGCA-3′) primers to confirm the identity of the pathogen. A voucher of the fungal strain was deposited in the Specialized Medical Mycology Center Culture Collection at the Federal University of Ceará under code CEMM 05–5-059.
Homogenates of lungs, spleen, and liver of all bats were removed from storage and assayed by immunodiffusion tests specific for H. capsulatum and C. posadasii antigens (12) (ID Antigen H & M and IDCF Antigen; Immy Immunodiagnostics, Inc., Norman, OK, USA) according to the manufacturer’s instructions. None of the homogenates showed positive reactions in H. capsulatum immunodiffusion tests. However, positive antibodies against Coccidioides spp. were found in 1 sample of lung from Glossophaga soricina bats. Positive antigen reactions were seen in homogenate liver samples from 2 animals, identified as G. soricina and Desmodus rotundus bats. These results suggest natural coccidioidal infection among the animals evaluated.
Positive C. perspicillata and G. soricina bats were captured in the same place, a deserted house in the urban area of Aracoiaba (4°21′59.1′′S and 38°48′51.9′′W) that has a semi-arid climate, with a rainy season from February through April and an average rainfall of 1,010.3 mm per year. The vampire bat, D. rotundus, was captured inside a cave in Ubajara (3°48′14.3′′S and 40°52′46.2′′W), a city characterized by a warm, subhumid tropical climate, with a rainy period from January through April and rainfall of 1,483.5 mm per year.
Coccidioides spp. can infect many mammal species (13). In this study, C. posadasii was isolated from the lungs of C. perspicillata bats, a colonial species that can cohabitate with different species of chiropterans (14,15). We propose 3 hypotheses for this finding. First, we hypothesize that the ability to travel long distances daily in their search for food and the social behavior of chiropterans may promote the acquisition and dispersion of C. posadasii. As a result, infected bats may have migrated from areas where Coccidioides infections are endemic and introduced the fungus in previously non–disease-endemic areas. A second hypothesis involves the possible existence of other animals that cohabitate with bats in artificial or natural shelters as the primary source of C. posadasii infections. Our third hypothesis is that climate changes in recent decades, mainly the increasing temperature in South America, along with the desertification process, which affects approximately one third of Ceará State, might have contributed to this unusual finding. Hypothetical links between climate changes and the epidemiology of other fungal diseases have been described. Studies need to be performed to investigate the role of chiropterans in the epidemiologic cycle of coccidioidomycosis.
Dr Cordeiro is professor of medical microbiology at Universidade Federal do Ceará, Brazil. Her research focuses on pathogenic fungi in humans and animals.
Acknowledgments
We thank the Secretaria de Saúde do Estado do Ceará for technical support.
This work was supported by grants from the National Scientific and Technological Development Council (CNPq–process no. 306637/2010-3 and Programa de Capacitação em Taxonomia 562296/2010-7).
References
- Wibbelt G, More MS, Schountz T, Voigt CC. Emerging diseases in Chiroptera: why bats? Biol Lett. 2010;6:438–40. DOIPubMedGoogle Scholar
- Chaturvedi V, Springer DJ, Behr MJ, Ramani R, Li X, Peck MK, Morphological and molecular characterizations of psychrophilic fungus Geomyces destructans from NewYork bats with white nose syndrome (WNS). PLoS ONE. 2010;5:e10783. DOIPubMedGoogle Scholar
- Emmons CW, Klite PD, Baer GM, Hill WB Jr. Isolation of Histoplasma capsulatum from bats in the United States. Am J Epidemiol. 1966;84:103–9.PubMedGoogle Scholar
- Galvão Dias MA, Zancopé Oliveira RM, Giudice MC, Montenegro Netto H, Jordão LR, Grigorio IM, Isolation of Histoplasma capsulatum from bats in the urban area of São Paulo State, Brazil. Epidemiol Infect. 2010. Epub ahead of print.
- Daher EF, Silva GB Jr, Barros FAS, Takeda CFV, Mota RMS, Ferreira MT, Clinical and laboratory features of disseminated histoplasmosis in HIV patients from Brazil. Trop Med Int Health. 2007;12:1108–15. DOIPubMedGoogle Scholar
- Sidrim JJC, Silva LCI, Nunes JMA, Rocha MFG, Paixão GC. Le nord-est Brésilien, région d’endémie de coccidioïdomycose? J Mycol Med. 1997;7:37–9.
- Wanke B, Lazera M, Monteiro PC, Lima FC, Leal MJ, Ferreira Filho PL, Investigation of an outbreak of endemic coccidioidomycosis in Brazil’s northeastern state of Piauí with a review of the occurrence and distribution of Coccidioides immitis in three other Brazilian states. Mycopathologia. 1999;148:57–67. DOIPubMedGoogle Scholar
- Cordeiro RA, Brilhante RSN, Rocha MFG, Bandeira SP, Fechine MAB, Camargo ZP, Twelve years of coccidioidomycosis in Ceará State, Northeast Brazil: epidemiologic and diagnostic aspects. Diagn Microbiol Infect Dis. 2010;66:65–72. DOIPubMedGoogle Scholar
- Cordeiro RA, Brilhante RSN, Rocha MFG, Fechine MAB, Camara LMC, Camargo ZP, Phenotypic characterization and ecological features of Coccidioides spp. from Northeast Brazil. Med Mycol. 2006;44:631–9. DOIPubMedGoogle Scholar
- Taylor ML, Chávez-Tapia CB, Vargas-Yañez R, Rodríguez-Arellanes G, Peña-Sandoval GR, Toriello C, Environmental conditions favoring bat infection with Histoplasma capsulatum in Mexican shelters. Am J Trop Med Hyg. 1999;61:914–9.PubMedGoogle Scholar
- Umeyama T, Sano A, Kamei K, Niimi M, Nishimura K, Uehara Y. Novel approach to designing primers for identification and distinction of the human pathogenic fungi Coccidioides immitis and Coccidioides posadasii by PCR amplification. J Clin Microbiol. 2006;44:1859–62. DOIPubMedGoogle Scholar
- Brilhante RSN, Cordeiro RA, Rocha MFG, Fechine MAB, Furtado FM, Nagao-Dias AT, Coccidioidal pericarditis: a rapid presumptive diagnosis by an in-house antigen confirmed by mycological and molecular methods. J Med Microbiol. 2008;57:1288–92. DOIPubMedGoogle Scholar
- Laniado-Laborin R. Expanding understanding of epidemiology of coccidioidomycosis in the Western Hemisphere. Ann N Y Acad Sci. 2007;1111:19–34. DOIPubMedGoogle Scholar
- Cloutier D, Thomas DW. Carollia perspicillata. Mamm Species. 1992;417:1–9.
- Alvarez J, Willing MR, Jones JK Jr, Webster D. Glossophaga soricina. Mamm Species. 1991;379:1–7. DOIGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 18, Number 4—April 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Rossana Cordeiro, 10 50 Av. Domingos Olimpio, Apto. 501, Bairro Jose Bonifacio, CEP 60040-080, Fortaleza, Ceará, BrazilRossana Cordeiro, 10 50 Av. Domingos Olimpio, Apto. 501, Bairro Jose Bonifacio, CEP 60040-080, Fortaleza, Ceará, Brazil
Top