Volume 21, Number 3—March 2015
Letter
Endemic and Imported Measles Virus–Associated Outbreaks among Adults, Beijing, China, 2013
Abstract
In 2013, a resurgence of measles occurred in Beijing, China. The outbreaks occurred among adults and were associated with endemic genotype H1 and imported genotype D8 viruses. Migrant workers were disproportionately represented in the outbreaks; thus, vaccinating such workers against measles may be an effective strategy toward the elimination of this disease.
All 6 World Health Organization (WHO) regions have set goals to eliminate measles (1,2). In China, a nationwide measles supplementary immunization activity was conducted in 2010, and the incidence of measles in mainland China subsequently reached its lowest reported level in 2012 (6,183 cases, 4.6 cases/million total population). However, in 2013, a nationwide resurgence of measles occurred primarily among young, unvaccinated children (3). In contrast to the nationwide resurgence, the measles resurgence in China’s capital, Beijing, was primarily among adults >15 years of age (65.7% of cases) and occurred in large, clothing wholesale markets.
Beijing has >12.96 million permanent residents and an additional ≈7.73 million floating residents (i.e., internal migrants who move into the city, usually for employment) (4). The routine measles vaccination schedule in use in Beijing consists of 3 doses of measles-containing vaccine; the first dose is administered at 8 months, the second at 18 months, and the third at 6 years of age. Also, since 2006, an additional dose has been administered to college students who move to Beijing to study (5). In this study, we used genotype analysis to describe the measles outbreaks among adults in Beijing, and we suggest an immunization strategy to help prevent similar outbreaks in the future.
In early 2013, a resurgence of measles in Beijing was reported to the China Information System for Disease Control and Prevention, Chinese Center for Disease Control and Prevention (Beijing); by the end of the year, a total of 1,233 suspected cases had been reported and investigated. Serum and throat swab samples were collected from 97.3% and 96.8% of the suspected case-patients, respectively. The samples were tested for measles IgM by using the VIRION SERION ELISA Measles Virus IgM test (Virion/Serion, Wurzburg, Germany) or for measles virus genes by using a real-time reverse transcription PCR kit (Jiangsu Bioperfectus Technologies, Jiangsu, China). Of the 1,233 samples, 558 were positive, and 5 additional cases were confirmed by epidemiologic linkage. Thus, a total of 563 measles cases in Beijing were confirmed in 2013; this number represents a 6-fold increase from the number of cases in 2012.
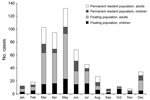
The number of reported cases was highest during March–May. Most cases occurred among a floating population of adult migrant workers and permanent adult residents and their children (Figure); 67.3% of the cases in adults were in migrant workers. Reports of cases increased shortly after the national holidays associated with the spring festival, during which many persons travel to visit relatives.
Among the persons with confirmed measles, 22.8% (128) were <8 months of age, 11.5% (65) were 8 months–14 years of age, and 65.7% (370) were >15 years of age and defined as adult patients. The median age of the adult patients was 23.5 years (range 15.0–70.0 years). Vaccination history was unknown for 87.6% (324/370) of the adults. Among the 65 patients in the age group targeted for measles vaccination, 36.9% (24) had not been vaccinated because of contraindications.
We used real-time reverse transcription PCR–positive samples to try to isolate and genotype virus from 468 of the 563 positive samples. Genomic sequencing and phylogenetic analyses were based on N450 nucleotide sequences, as previously described (6–8). Measles genotype was determined for 45.6% (257/563) of the cases reported from 14 of 16 districts or counties. Among those 257 cases, 84.0% (216) were caused by endemic genotype H1 viruses and 15.2% (39) and 0.78% (2), respectively, were caused by imported genotype D8 and D9 viruses. All 14 districts had confirmed measles cases caused by genotype H1 viruses, and 6 and 2 districts, respectively, had cases caused by genotype D8 and D9 viruses. Of the 257 measles cases with genotype information, 61.6% (133) of those caused by genotype H1 and 97.4% (38) of those caused by genotype D8 were in adults (Table).
Measles viruses within a transmission chain had identical or nearly identical N450 sequences (9). Phylogenetic analysis showed that 216 genotype H1 viruses were associated with 30 different chains of transmission (Technical Appendix Figure, panels A, B), and 39 genotype D8 viruses were associated a single chain of transmission, although 1 virus differed by only 1 nt (Technical Appendix Figure, panels A, C). Nucleotide sequences of 43 representative viruses were deposited in GenBank (accession nos. KJ556851–93). A search of the World Health Organization’s Measles Nucleotide Surveillance (MeaNS) database (10) showed that genomic sequences of genotype D8 viruses from the outbreak shared 99.8%–100% nucleotide identity with genomic sequences of strains from measles patients in Russia, France, Canada, Thailand, Denmark, Germany, and other locations.
Genotype D8 measles viruses were associated with at least 2 outbreaks in different large, wholesale clothing markets. The outbreaks occurred during March–July 2013 and were almost completely confined to adults; only 1 child was infected, possibly because of high coverage of measles-containing vaccine among Beijing children. We were unable to identify the source of the infections. Phylogenetic analysis suggested that the genotype D8 virus might have been imported to Beijing from Europe, America, or another location (10–13) and subsequently spread beyond Beijing by virus transmission from infected adults (data not shown). For at least 21 years, genotype H1 measles viruses have been the only endemic measles circulating in China (6–8); measles cases caused by all other genotypes have been associated with imported viruses (7).
Our findings show that transmission of measles virus among adults contributed to a resurgence of measles in Beijing during 2013. Nonendemic genotype D8 measles viruses were associated with at least 2 outbreaks in different wholesale clothing markets during March–July, 2013. Many persons from domestic and international areas visit wholesale markets every day; thus, such markets are high-risk settings for the transmission and importation of measles viruses (14).
Because migrant workers were disproportionately affected in the Beijing outbreaks and because their work settings have high measles transmission potential, we support an outreach strategy to prevent measles among this floating population. These workers usually live with their families and register with the local authorities for government services. Thus, we suggest that the offer of measles vaccine to workers as they register to live and work in the commodity markets might be a reasonable strategy to prevent future measles outbreaks. Serologic surveys can be used to refine such a strategy by assessing immunity within the target population.
The foundation strategy for eliminating measles globally is based on the timely vaccination of young children with 2 valid doses of measles-containing vaccine. However, laboratory-supported surveillance analysis and outbreak investigations are critical to the identification of gaps in immunity among older age groups, which may need to be filled, and to the identification of strategies to prevent similar outbreaks. The fact that more than a third of infected children in the vaccine-targeted age group were unvaccinated because of vaccination contraindications suggests that an evaluation is needed to ensure the use of valid contraindications only.
It is difficult to identify narrow, age-based risk groups to target for vaccination when a high proportion of adults are unvaccinated and may still be susceptible to measles. Unselective vaccination of millions of adults, based solely on their age, is unlikely to be feasible in China. Additional risk factors for measles need to be identified to develop more feasible immunization strategies.
Dr. Chen is an associate researcher at the Beijing Center for Disease Control and Prevention. Her major research interests are the epidemiology and molecular epidemiology of respiratory viruses.
Acknowledgments
We thank the district and county measles laboratory staff and epidemiologists in Beijing for providing clinical specimens, isolates, and epidemiologic data. We also acknowledge anonymous reviewers for their comments that improved the manuscript.
This work is supported by grants from the National Natural Science Foundation of China (no. 81371791), the Key Technologies R&D Program of the National Ministry of Science (nos. 2012ZX10004215, 2013ZX10004-202, 2012ZX10004201-003, and 2012ZX10004-206), World Health Organization Measles Regional Reference Laboratory funding, and World Health Organization funding (no. WP CHN AAA 011 XZ 08).
References
- Perry RT, Gacic-Dobo M, Dabbagh A, Mulders MN, Strebel PM, Okwo-Bele JM, Global control and regional elimination of measles, 2000–2012. MMWR Morb Mortal Wkly Rep. 2014;63:103–7 .PubMedGoogle Scholar
- Strebel PM, Cochi SL, Hoekstra E, Rota PA, Featherstone D, Bellini WJ, A world without measles. J Infect Dis. 2011;204(Suppl 1):S1–3. DOIPubMedGoogle Scholar
- Ma C, Hao L, Zhang Y, Su Q, Rodewald L, An Z, Monitoring progress towards the elimination of measles in China: an analysis of measles surveillance data. Bull World Health Organ. 2014;92:340–7. DOIPubMedGoogle Scholar
- Beijing Statistical Information Net. Beijing 2013 National Economic and Social Development Statistics Bulletin [in Chinese] [cited 2014 Dec 19]. http://www.bjstats.gov.cn/xwgb/tjgb/ndgb/201402/t20140213_267744.htm
- Li J, Lu L, Pang X, Sun M, Ma R, Liu D, A 60-year review on the changing epidemiology of measles in capital Beijing, China, 1951–2011. BMC Public Health. 2013;13:986. DOIPubMedGoogle Scholar
- Zhang Y, Xu S, Wang H, Zhu Z, Ji Y, Liu C, Single endemic genotype of measles virus continuously circulating in China for at least 16 years. PLoS ONE. 2012;7:e34401. DOIPubMedGoogle Scholar
- Zhang Y, Wang H, Xu S, Mao N, Zhu Z, Shi J, Monitoring progress toward measles elimination by genetic diversity analysis of measles viruses in China 2009–2010. Clin Microbiol Infect. 2014;20:O566–77 . DOIPubMedGoogle Scholar
- Xu W, Tamin A, Rota JS, Zhang L, Bellini WJ, Rota PA. New genetic group of measles virus isolated in the People’s Republic of China. Virus Res. 1998;54:147–56. DOIPubMedGoogle Scholar
- Rota PA, Brown K, Mankertz A, Santibanez S, Shulga S, Muller CP, Global distribution of measles genotypes and measles molecular epidemiology. J Infect Dis. 2011;204(Suppl 1):S514–23. DOIPubMedGoogle Scholar
- World Health Organization. Measles Nucleotide Surveillance (MeaNS) database [cited 2014 Oct 23]. http://www.who-measles.org/Public/Web_Front/main.php
- Metin O, Tanir G, Oz FN, Kalaycioglu AT, Yolbakan S, Tuygun N, Evaluation of 44 pediatric measles cases detected in Ankara, Turkey during 2012–2013 epidemic and molecular characterization of the viruses obtained from two cases [in Turkish]. Mikrobiyol Bul. 2014;48:259–70. DOIPubMedGoogle Scholar
- Salimi V, Abbasi S, Zahraei SM, Fatemi-Nasab G, Adjaminezhad-Fard F, Shadab A, Implementation of a national measles elimination program in Iran: phylogenetic analysis of measles virus strains isolated during 2010–2012 outbreaks. PLoS ONE. 2014;9:e94846. DOIPubMedGoogle Scholar
- Baugh V, Figueroa J, Bosanquet J, Kemsley P, Addiman S, Turbitt D. Ongoing measles outbreak in Orthodox Jewish community, London, UK. Emerg Infect Dis. 2013;19:1707–9. DOIPubMedGoogle Scholar
- Burgess CPMP, Skov S, Dowse G. Measles transmission by “fly-in fly-out” workers in Australia. Aust N Z J Public Health. 2013;37:423–6. DOIPubMedGoogle Scholar
Figure
Table
Cite This Article1These authors contributed equally to this article.
Related Links
Table of Contents – Volume 21, Number 3—March 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Wenbo Xu, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, 155# Changbai Rd, Changping District, Beijing, China, 102206; ; or Ying Deng, Beijing Center for Diseases Prevention and Control, 16# Hepingli Zhongjie, Chaoyang District, Beijing, 100000, China
Top