Volume 21, Number 6—June 2015
Research
Sequence Type 4821 Clonal Complex Serogroup B Neisseria meningitidis in China, 1978–2013
Abstract
Serogroup B Neisseria meningitidis strains belonging to sequence type 4821 clonal complex (CC4821), a hyperinvasive lineage first identified for serogroup C in 2003, have been increasingly isolated in China. We characterized the outer membrane protein genes of 48 serogroup B and 214 serogroup C strains belonging to CC4821 and analyzed the genomic sequences of 22 strains. Four serogroup B strains had porin A (i.e., PorA), PorB, and ferric enterobactin transport (i.e., FetA) genotypes identical to those for serogroup C. Phylogenetic analysis of the genomic sequences showed that the 22 CC4821 strains from patients and healthy carriers were unevenly clustered into 2 closely related groups; each group contained serogroup B and C strains. Serogroup B strains appeared variable at the capsule locus, and several recombination events had occurred at uncertain breakpoints. These findings suggest that CC4821 serogroup C N. meningitidis is the probable origin of highly pathogenic CC4821 serogroup B strains.
Neisseria meningitidis bacteria are a leading cause of bacterial meningitis and other serious invasive bacterial infections. Among the 12 identified serogroups, A, B, C, Y, W, and X are responsible for most invasive meningococcal diseases. The geographic distribution and epidemic capabilities of N. meningitidis differ according to serogroup (1). On the basis of the epidemiology of N. meningitidis, many countries have included different formulations of the meningococcal vaccine in their routine immunization programs (2–4). These vaccines have significantly reduced the incidence of meningococcal diseases (5,6). However, in several countries, the introduction of vaccines targeting specific serogroups may have led to the replacement of vaccine serogroups by other, nonvaccine, serogroups (7–11). Serogroup replacement can occur as a result of capsule switching (3,8,12–19) or as a result of importation of a serogroup meningococcus from other regions (20).
In the past century in China, most meningococcal epidemics were caused by strains of N. meningitidis that belonged to sequence type 1 (ST-1) and ST-5 clonal complexes (CC1 and CC5, respectively) (21). Therefore, beginning in the early 1980s, a polysaccharide vaccine against serogroup A was incorporated into the routine immunization program. Use of this vaccine led to a significant decrease in the incidence of meningococcal diseases (22). However, in 2003, a serogroup C outbreak caused by a CC4821 strain was reported in China; this clonal lineage had not been detected in other countries (23). CC4821 corresponding to serogroup C has subsequently become one of the dominant lineages in China (21). To combat this serogroup replacement, several vaccines were developed against serogroup C or serogroups A and C. During 2005–2010, subsequent to the time when serogroup C and A N. meningitidis infections had been prevalent, cases caused by serogroup W strains belonging to CC11 began increasing in China (24,25). In addition to these 3 serogroups, serogroup B strains have been isolated from patients and healthy carriers. Serogroup B strains showed high genetic diversity and were usually associated with sporadic infections (21). Some of the prevalent clonal lineages that are common in many countries (e.g., CC32 and CC41/44) (26) are rarely isolated in China (21). However, CC4821 became a dominant lineage among serogroup B strains since they were first identified in 2005. When we retrospectively studied the strains in our collection, CC4821 strains were isolated as early as in 1978, and the lineage included serogroup B and C strains (27). Nevertheless, few CC4821 strains were isolated during 1970–1980, and no CC4821-related outbreaks were identified during that time (Z. Shao, unpub. data).
Capsular switching between N. meningitidis serogroups B and C is frequently observed (3,7,8,12,16–19); therefore, serogroups B and C most likely have similar DNA sequences in the capsule locus, leading to increased horizontal DNA transfer between these serogroups (16). We propose that capsular switching occurred between the CC4821 serogroup B and C N. meningitidis strains. To elucidate the relationship between them, we investigated the epidemiology of CC4821 serogroup B strains, characterized the outer membrane protein (OMP) genes of these strains, and analyzed the genome sequences and capsule locus sequences of specific strains.
Meningococcal Meningitis Surveillance in China
A population-based surveillance system for meningococcal meningitis exists throughout China. Provincial Center for Disease Control and Prevention (CDC) staff routinely collect strains suspected to be N. meningitidis on the basis of morphologic and biochemical characteristics, and they periodically conduct surveys of N. meningitidis carriers for outbreak investigation, surveillance, and research purposes. If no strain is isolated, clinical specimens are collected by the provincial CDC. The strains and specimens are sent to the China CDC national reference laboratory for identification, or they are tested at the provincial CDC, and results are sent to China CDC. Our laboratory identifies strains and performs multilocus sequence typing (MLST) on confirmed N. meningitidis strains.
Meningococcal Strains and DNA Preparation
Forty-eight serogroup B and 214 serogroup C N. meningitidis strains previously assigned to CC4821 were included in this study. These strains were collected from 20 provinces in China during 1978–2013. Among the 48 serogroup B strains, 9 were from patients and 39 were from asymptomatic carriers. Among the 214 serogroup C strains, 91 were from patients and 123 were from asymptomatic carriers. One strain was identified as serogroup B by PCR; serogroups for the other strains were determined by slide agglutination with specific rabbit antisera (Remel Europe Ltd, Kent, UK) (Technical Appendix Table 1).
The selected strains were propagated on single plates containing Columbia agar in a 5% CO2 atmosphere at 37°C for 18 h. Genomic DNA was extracted by using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Sequencing of OMP Genes
The porin A (porA), porB, and ferric enterobactin transport (fetA) genes were amplified from freshly prepared DNA. The PCR and sequencing were performed as previously described (28–30).
Genome Sequences of Meningococcal Strains
Eight serogroup B and 14 serogroup C N. meningitidis CC4821 strains were sequenced by constructing 2 paired-end libraries with average insert lengths of 500 bp. The sequences were generated by using an Illumina HiSeq 2000 sequencing platform (Illumina, San Diego, CA, USA) and assembled into contigs and scaffolds by using SOAPdenovo, release 1.04 (http://soap.genomics.org.cn/soapdenovo.html). Genes were predicted by using Glimmer (31) with default parameters and then annotated by sequence comparisons with nucleotide and nonredundant protein sequence databases and the SwissProt (http://web.expasy.org/docs/swiss-prot_guideline.html) database by using BLAST (http://blast.ncbi.nlm.nih.gov) with an e-value of 1e−5. The genome sequence data obtained in this study were submitted to GenBank under the accession numbers JMBH00000000, JMCN00000000–JMCZ00000000, JMDA00000000–JMDH00000000. The complete reference genome sequences of multiple N. meningitidis strains and 1 N. lactamica strain were downloaded from the Completed Genomic Sequence section of the publicly available Entrez Genome database (http://www.ncbi.nlm.nih.gov/genomes/static/EG_T.html).
Phylogenetic Analysis of N. meningitidis Genome Sequences
All 22 CC4821 genomes and 14 reference N. meningitidis genomes were used to construct a phylogenetic tree, and the genome of N. lactamica strain 020-06 was used as the outgroup. We identified the core genes in these 37 genomes in 2 steps. First, we used OrthoMCL (http://orthomcl.org/orthomcl/) to cluster all genes into orthologous groups and then selected the groups that were shared by all 37 genomes. Second, we removed the orthologous groups associated with known mobile genetic elements, such as genomic islands, phages, and transposons. The remaining orthologous groups were considered to be core genes. For all 37 genomes, the amino acid sequences of the core genes were concatenated, and multiple sequence alignments were performed by using MUSCLE (http://www.ebi.ac.uk/Tools/msa/muscle/). We then constructed a phylogenetic tree using the neighbor-joining method with MEGA4 (http://www.megasoftware.net/).
Identification and Analysis of Capsule Locus
The contigs containing capsule locus sequences were compared with the N. meningitidis complete reference genome sequences by using blastn (http://blast.ncbi.nlm.nih.gov) to identify orthologous sequences and determine their levels of similarity. For contigs with a gap within the UDP-glucose 4-epimerase (galE) gene, PCR and Sanger sequencing were performed to close the gap. The capsule locus between genes transcriptional accessory protein (tex) and galE of CC4821 serogroup B strains were then aligned with the corresponding sequences from all serogroup B complete genomes by using MUSCLE version 3.6. On the basis of the alignment results, we used the genome with the highest level of similarity with CC4821 serogroup B strains as the reference sequence in subsequent analyses. To reveal the relationship between CC4821 serogroups B and C, we aligned the capsule locus genes from all study strains with those of serogroup C isolate 053442 (ST-4821) and the selected serogroup B reference strain.
Identification of Recombination Breakpoints
We compared the capsule locus between genes tex and galE from study and reference strains by using MUSCLE. We analyzed recombination events within the capsule locus sequences by using different methods in RDP (Recombination Detection Program) version 4 beta 27 (http://web.cbio.uct.ac.za/~darren/rdp.html) with default parameters.
Epidemiology of CC4821 Serogroup B Strains
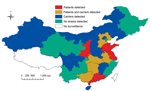
During the 1970s and 1980s, a total of 12 CC4821 serogroup B and C strains (6 from each serogroup) were collected from 6 provinces in China: Hebei, Henan, Jiangxi, Liaoning, Shanxi, and Shanghai. During March 2005–March 2013, N. meningitidis strains belonging to CC4821 serogroup B were isolated from the cerebrospinal fluid or blood samples of meningococcal patients in 10 provinces and from pharyngeal swab specimens from healthy carriers in 9 other provinces in China. These 19 provinces represent diverse geographic and climate conditions, and the cases were not related. (Figure 1)
OMP Genotype Profiles
The porA gene was sequenced for all studied strains. The genotype profiles revealed a high degree of diversity even among strains with identical STs. Serogroup B N. meningitidis strains had 26 PorA genotypes, and serogroup C strains had 16. Among these, 8 genotypes were detected among both serogroup B and C strains, representing 35.4% of serogroup B and 77.6% of serogroup C strains. P1.7-2, 14 was the predominant genotype in serogroups C (55.6%) and B (12.5%). Two combinations of ST and PorA genotype were observed in serogroup C and B strains: ST-4821: P1.7-2, 14 and ST-4821: P1.20, 23-1. In total, 4 serogroup B strains had combination genotypes that were the same as those for serogroup C strains (Technical Appendix Table 2). To elucidate the relationship between the serogroup B and C strains, we sequenced the porB and fetA genes for strains with the combination genotype ST-4821: P1.7-2, 14 or ST-4821: P1.20, 23-1. The sequencing analysis showed that all 4 serogroup B strains had identical PorB and FetA genotypes (3-48 and F3-3, respectively), which were also the main PorB and FetA genotypes in the serogroup C strains.
Phylogenetic Analysis of Genome Sequences
A total of 1,200 core genes (385,358 aa, corresponding to 50.9% of the genome of serogroup B N. meningitidis strain MC58) were selected for the phylogenetic analysis as described in Materials and Methods. The neighbor-joining phylogenetic tree reconstructed from the concatenated amino acid sequences of these genes showed that the 22 CC4821 strains were clustered into 2 closely related groups (groups I and II), which were distantly related to the N. meningitidis reference strains belonging to other CCs (Figure 2). Both groups contained serogroup B and serogroup C strains, although there were more serogroup C strains in group I and more serogroup B strains in group II. Each group consisted of 11 strains, but there were more invasive strains in group I (n = 9) than in group II (n = 4). The reference strain 053442, which was isolated from a patient and belonged to CC4821, was clustered with group I. Considering the genetic distances between the strains, the strains in group II were less closely related to each other than those in group I.
Analysis of Capsular Locus
The capsule locus sequences between genes tex and galE were retained from 20 sequenced CC4821 genomes (12 serogroup C and 8 serogroup B). For 2 strains, there were gaps within galE or ctrA gene.
The DNA sequences of the capsular locus were compared with the homologous gene clusters of the reference strains (053442, serogroup C, ST-4821; H44-76, serogroup B, ST-32) (Figure 3). All capsular locus genes of 9 serogroup C strains (group I) were 99.9%–100% identical to that for reference strain 053442. The other 3 serogroup C strains (group II) were less similar to 053442 at the genes upstream of polysialic acid capsule export outer-membrane lipoprotein (ctrA) and downstream of sialic acid synthase (siaC). All serogroup B strains had polysialyltransferase (siaD) genes that were distinct from that for reference strain 053442 and did not contain the O-acetyltransferase (oatC) gene between genes siaD and open-reading frame 2. With the exception of 1 serogroup B strain (341215, in group I), which shared high similarity (99.8%–100%) with 053442 at the genes upstream of siaD, the serogroup B strains had low similarity at ctrC and preceding genes. Serogroup B and C strains shared <99% similarity with strain H44-76 at genes tex, ctrD, ctrC, siaA, open-reading frame 2, and galE. However, siaD genes of the serogroup B strains and H44-76 shared >99.7% similarity.
We used RDP, GENECONV (http://www.math.wustl.edu/~sawyer/geneconv/), BootScan (32), and the 3seq method (33) to analyze the recombination events within the capsule locus, but failed to obtain consistent results about the breakpoint. Nevertheless, all the results indicated that different and multiple events had occurred at the capsule locus within and among strains (Figure 4). Compared with strains belonging to group II, those belonging to group I (genome-based phylogenetic tree) had fewer recombination events, and most of the events were within the same serogroup.
Phylogenetic analysis of the N. meningitidis core genome amino acid sequences showed that all 22 sequenced CC4821 strains were closely related, irrespective of serogroup, ST, and PorA type, which indicated capsular switching between serogroups C and B. However, serogroups B and C were detected in both phylogenetic groups, so the direction of capsule switching remains to be determined (34). The multiple recombination events may have occurred within the capsule locus, which explains why we could not define the recombination breakpoints at the capsule locus. The present evidence is not sufficient to confirm whether capsular switching occurred from serogroup C to B or vice versa. Further study is required to examine how the capsular switching occurred.
In previous studies of N. meningitidis capsule switching, MLST and OMP genotyping were used to characterize the relationship between the new variants and the candidate parental organisms (3,7,8,16,18,19). If the molecular profiles (e.g., ST, PorA, and FetA) are identical or highly similar between 2 different serogroups, capsule switching is inferred. In our study, only 4 ST-4821 serogroup B N. meningitidis strains were observed to share the same STs and OMP gene types (PorA, PorB, and FetA) with serogroup C N. meningitidis. However, analysis of the core genome sequencing results showed that all serogroup B strains, even those with specific profiles, are closely related to serogroup C strains. Horizontal DNA transfer occurs frequently between N. meningitidis strains. Recombination events involving the MLST locus and the porA, porB, and fetA genes would impede the analysis of capsule switching and might lead to false conclusions (15). An analysis based on genomic sequence can reveal the relationships among strains more accurately, so additional genome analysis is required to resolve the observed discrepancies.
CC4821 serogroup C N. meningitidis had been the dominant lineage in China for a decade (2003–2014), although the incidence of invasive disease remained at a moderate level (<0.1 case/100,000 population; Z. Shao, unpub. data) because of mass vaccination. The emergence and circulation of CC4821 serogroup B N. meningitidis might increase the incidence of invasive meningococcal diseases and even cause epidemics and outbreaks in China. This potential risk can be attributed to the CC4821 lineage itself and to the particularity of vaccine against serogroup B N. meningitidis. Since the first outbreak occurred in 2003, the CC4821 serogroup C N. meningitidis epidemic has rapidly involved most provinces of China. This epidemic indicates that CC4821 N. meningitidis had substantial ability to spread extensively and cause invasive disease. The core genome–based phylogenetic analysis showed that CC4821 strains from patients and healthy carriers were unevenly clustered into 2 groups, suggesting a difference in pathogenicity between these 2 groups of strains. Furthermore, group I, which possessed more patient-derived strains, was the most recent clade on the tree, and the strains in this group were more closely related to each other than those in group II, indicating that group I was a highly invasive sublineage of CC4821. Because of the emergence of serogroup B strains belonging to this highly invasive N. meningitidis CC4821 sublineage, we must remain alert for a potential epidemic. An effective protective vaccine specifically against the serogroup B capsule polysaccharide does not exist (35). Although several vaccines consisting of specific proteins have been licensed for use against serogroup B infection (36), their effectiveness varies by clonal lineage, and their effectiveness has not been studied in China. This critical public health concern highlights the need for further epidemiologic surveillance to monitor changes in the incidence of meningococcal disease caused by N. meningitidis CC4821 serogroup B and for improved public health disease control strategies in the future.
Circulation of the CC4821 clonal lineage has not been observed in other regions, even though it is hyperendemic in China. The reason for this limited distribution is not readily apparent, which highlights the need for continued surveillance. The virulence and pathogenic mechanisms of this newly identified hyperinvasive lineage are not well understood (23). Comparative genome analysis within CC4821 strains and those from other CCs may help to identify potential additional virulence factors of N. meningitidis. In China, mass vaccination against meningococcal disease targets only N. meningitidis serogroups A and C. Thus, monitoring the appearance and spread of CC4821 in other serogroups is important because capsule switching among N. meningitidis serogroups C, B, W, and Y has been observed in several countries (14,16).
Dr. Zhu is a research scientist at the State Key Laboratory for Infectious Disease Prevention and Control, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. Her primary research interest is the variation and prevalence of N. meningitidis bacteria.
Acknowledgment
This work was supported by grants from the National Key Program for Infectious Disease of China (contract no. 2013ZX10004221) and the National Basic Research Program of China (973 Program, 2011CB504900).
References
- Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Global epidemiology of invasive meningococcal disease.Popul Health Metr.2013;11:17. DOIPubMedGoogle Scholar
- Hepkema H, Pouwels KB, van der Ende A, Westra TA, Postma MJ. Meningococcal serogroup A, C, W135 and Y conjugated vaccine: a cost-effectiveness analysis in the Netherlands.PLoS ONE.2013;8:e65036. DOIPubMedGoogle Scholar
- Alcalá B, Arreaza L, Salcedo C, Uria MJ, De La Fuente L, Vazquez JA. Capsule switching among C:2b:P1.2,5 meningococcal epidemic strains after mass immunization campaign, Spain.Emerg Infect Dis. 2002;8:1512–4. DOIPubMedGoogle Scholar
- Bilukha OO, Rosenstein N; Centers for Disease Control and Prevention. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2005;54:1–21.PubMedGoogle Scholar
- Salleras L, Dominguez A, Cardenosa N. Dramatic decline of serogroup C meningococcal disease in Catalonia (Spain) after a mass vaccination campaign with meningococcal C conjugated vaccine.Vaccine.2003;21:729–33. DOIPubMedGoogle Scholar
- Ramsay ME, Andrews N, Kaczmarski EB, Miller E. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England.Lancet.2001;357:195–6. DOIPubMedGoogle Scholar
- Lancellotti M, Guiyoule A, Ruckly C, Hong E, Alonso JM, Taha MK. Conserved virulence of C to B capsule switched Neisseria meningitidis clinical isolates belonging to ET-37/ST-11 clonal complex.Microbes Infect. 2006;8:191–6. DOIPubMedGoogle Scholar
- Castiñeiras TM, Barroso DE, Marsh JW, Tulenko MM, Krauland MG, Rebelo MC, Capsular switching in invasive Neisseria meningitidis, Brazil.Emerg Infect Dis. 2012;18:1336–8. DOIPubMedGoogle Scholar
- Pedro LG, Boente RF, Madureira DJ, Matos JA, Rebelo CM, Igreja RP, Diagnosis of meningococcal meningitis in Brazil by use of PCR.Scand J Infect Dis. 2007;39:28–32. DOIPubMedGoogle Scholar
- MacNeil JR, Medah I, Koussoube D, Novak RT, Cohn AC, Diomande FV, Neisseria meningitidis serogroup W, Burkina Faso, 2012.Emerg Infect Dis. 2014;20:394–9. DOIPubMedGoogle Scholar
- Pérez-Trallero E, Vicente D, Montes M, Cisterna R. Positive effect of meningococcal C vaccination on serogroup replacement in Neisseria meningitidis.Lancet.2002;360:953. DOIPubMedGoogle Scholar
- Swartley JS, Marfin AA, Edupuganti S, Liu LJ, Cieslak P, Perkins B, Capsule switching of Neisseria meningitidis.Pro cNatl Acad Sci U S A. 1997;94:271–6. DOIPubMedGoogle Scholar
- Wang Q, Shao Z, Wang X, Gao Y, Li M, Xu L, Genetic study of capsular switching between Neisseria meningitidis sequence type 7 serogroup A and C strains.Infect Immun. 2010;78:3883–8. DOIPubMedGoogle Scholar
- Tsang RS, Law DK, Tyler SD, Stephens GS, Bigham M, Zollinger WD. Potential capsule switching from serogroup Y to B: the characterization of three such Neisseria meningitidis isolates causing invasive meningococcal disease in Canada.Can J Infect Dis Med Microbiol.2005;16:171–4.PubMedGoogle Scholar
- Beddek AJ, Li MS, Kroll JS, Jordan TW, Martin DR. Evidence for capsule switching between carried and disease-causing Neisseria meningitidis strains.Infect Immun. 2009;77:2989–94. DOIPubMedGoogle Scholar
- Harrison LH, Shutt KA, Schmink SE, Marsh JW, Harcourt BH, Wang X, Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era—United States, 2000–2005.J Infect Dis. 2010;201:1208–24. DOIPubMedGoogle Scholar
- Castilla J, Vázquez JA, Salcedo C, Garcia Cenoz M, Garcia Irure JJ, Torroba L, B:2a:p1.5 meningococcal strains likely arisen from capsular switching event still spreading in Spain.J Clin Microbiol.2009;47:463–5. DOIPubMedGoogle Scholar
- Stefanelli P, Fazio C, Neri A, Sofia T, Mastrantonio P. First report of capsule replacement among electrophoretic type 37 Neisseria meningitidis strains in Italy.J Clin Microbiol.2003;41:5783–6. DOIPubMedGoogle Scholar
- Simões MJ, Cunha M, Almeida F, Furtado C, Brum L. Molecular surveillance of Neisseria meningitidis capsular switching in Portugal, 2002–2006.Epidemiol Infect. 2009;137:161–5. DOIPubMedGoogle Scholar
- Parent du Chatelet I, Barboza P, Taha MK. W135 invasive meningococcal infections imported from sub-Saharan Africa to France, January to April 2012.Euro Surveill.2012;17:pii20181.PubMedGoogle Scholar
- Zhou H, Gao Y, Xu L, Li M, Li Q, Li Y, Distribution of serogroups and sequence types in disease-associated and carrier strains of Neisseria meningitidis isolated in China between 2003 and 2008.Epidemiol Infect. 2012;140:1296–303. DOIPubMedGoogle Scholar
- Hu X. Surveillance and prevention of meningococcal diseases.China Journal of Public Health.2004;20:638–40.
- Shao Z, Li W, Ren J, Liang X, Xu L, Diao B, Identification of a new Neisseria meningitidis serogroup C clone from Anhui Province, China.Lancet.2006;367:419–23. DOIPubMedGoogle Scholar
- Shao Z, Zhou H, Gao Y, Ren H, Xu L, Kan B, Neisseria meningitidis serogroup W135, China.Emerg Infect Dis. 2010;16:348–9. DOIPubMedGoogle Scholar
- Zhou H, Liu W, Xu L, Deng L, Deng Q, Zhuo J, Spread of Neisseria meningitidis serogroup W clone, China.Emerg Infect Dis. 2013;19:1496–9. DOIPubMedGoogle Scholar
- Chang Q, Tzeng YL, Stephens DS. Meningococcal disease: changes in epidemiology and prevention.Clin Epidemiol.2012;4:237–45.PubMedGoogle Scholar
- Yang L, Shao Z, Zhang X, Xu L, Peng J, Xu X, Genotypic characterization of Neisseria meningitidis serogroup B strains circulating in China.J Infect. 2008;56:211–8. DOIPubMedGoogle Scholar
- Russell JE, Jolley KA, Feavers IM, Maiden MC, Suker J. PorA variable regions of Neisseria meningitidis.Emerg Infect Dis. 2004;10:674–8. DOIPubMedGoogle Scholar
- Urwin R, Kaczmarski EB, Guiver M, Fox AJ, Maiden MC. Amplification of the meningococcal porB gene for non-culture serotype characterization.Epidemiol Infect. 1998;120:257–62. DOIPubMedGoogle Scholar
- Thompson EA, Feavers IM, Maiden MC. Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component.Microbiology.2003;149:1849–58. DOIPubMedGoogle Scholar
- Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with Glimmer.Bioinformatics.2007;23:673–9. DOIPubMedGoogle Scholar
- Salminen MO, Carr JK, Burke DS, McCutchan FE. Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning.AIDS Res Hum Retroviruses. 1995;11:1423–5. DOIPubMedGoogle Scholar
- Boni MF, Posada D, Feldman MW. An exact nonparametric method for inferring mosaic structure in sequence triplets.Genetics.2007;176:1035–47. DOIPubMedGoogle Scholar
- Rishishwar L, Katz LS, Sharma NV, Rowe L, Frace M, Dolan Thomas J, Genomic basis of a polyagglutinating isolate of Neisseria meningitidis.J Bacteriol.2012;194:5649–56. DOIPubMedGoogle Scholar
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease.Vaccine.2009;27(Suppl 2):B51–63. DOIPubMedGoogle Scholar
- Caesar NM, Myers KA, Fan X. Neisseria meningitidis serogroup B vaccine development.MicrobPathog. 2013;57:33–40. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 21, Number 6—June 2015
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Zhujun Shao, National Institute for Communicable Disease Control and Prevention, Department of Respiratory Infectious Diseases, PO Box 5, Changping, Beijing 102206, China
Top