Volume 21, Number 6—June 2015
Dispatch
Prospective Multicenter International Surveillance of Azole Resistance in Aspergillus fumigatus
Abstract
To investigate azole resistance in clinical Aspergillus isolates, we conducted prospective multicenter international surveillance. A total of 3,788 Aspergillus isolates were screened in 22 centers from 19 countries. Azole-resistant A. fumigatus was more frequently found (3.2% prevalence) than previously acknowledged, causing resistant invasive and noninvasive aspergillosis and severely compromising clinical use of azoles.
Azole resistance is increasingly recognized as a problem in aspergillus diseases (1). Within the Aspergillus fumigatus species complex, new sibling species have been reported to cause invasive aspergillosis; these species are generally intrinsically less susceptible than A. fumigatus sensu strictu to azole compounds (2). Acquired resistance to azoles in A. fumigatus has become a public health concern because of the presumed fungicide-driven route of resistance selection and the associated risk for geographic migration. Surveillance studies show that, in areas to which Aspergillus is endemic, the environmental route of resistance selection contributes to >90% of resistance mechanisms in azole-resistant aspergillus diseases (1,3). Azole resistance has been observed in patients with no recent history of azole therapy, and the mortality rate for patients with azole-resistant invasive aspergillosis was 88% (3).
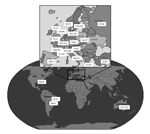
Our objective was to investigate the prevalence of azole resistance in clinical Aspergillus isolates. A multicenter international surveillance network was established (Surveillance Collaboration on Aspergillus Resistance in Europe [SCARE-network]), comprising 22 centers from 19 countries (18 European and 4 non-European sites) (Figure 1). To detect azole-resistant A. fumigatus, we developed a phenotypic screening-method using a 4-well plate format with agar supplemented with itraconazole, voriconazole, and posaconazole (4). Each center was asked to screen for azole resistance for 12 consecutive months. For each screened isolate, patient characteristics were registered through an online questionnaire, and patients with invasive aspergillosis were classified according to the European Organization for the Research and Treatment of Cancer/Mycoses Study Group consensus definitions (5).
For every A. fumigatus isolate that grew on any of the azole-containing wells, the primary culture isolate was sent both to the Radboud University Medical Centre (Nijmegen, the Netherlands) and Statens Serum Institute (Copenhagen, Denmark) for molecular species identification, susceptibility testing according to the EUCAST (European Committee on Antimicrobial Susceptibility Testing) broth microdilution reference method (6), and determination of the full coding sequence of both strands of the cyp51A gene and the promoter region by PCR amplification. For every resistant isolate, a susceptible control isolate was assigned; this control isolate was the first susceptible isolate screened on the 4-well plate format in the same center after the resistant isolate, and they received molecular species identification and susceptibility testing according to the EUCAST broth microdilution reference method (6).
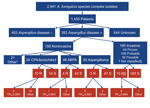
During January 2009–January 2011, a total of 3,788 Aspergillus isolates were screened for azole resistance by using the 4-well plates (Figure 1). Clinical information was available from 1,911 patients from 21 centers in 18 countries. Most (2,941 [77.6%]) isolates were classified as A. fumigatus species complex and were recovered from 1,450 patients. The most common underlying disease was chronic lung disease (30.0%), followed by cystic fibrosis (22.1%) and hemato-oncologic diseases (12.9%). A total of 204 (14.1%) patients had undergone hematopoietic stem cell transplantation or solid organ transplantation, and 265 (18.3%) had been treated with corticosteroids within 3 months before culture of the isolate. A total of 223 (15.4%) of 1,450 patients had received antifungal drugs within 3 months before, or at the time of the positive culture. For 806 (55.6%) patients, the clinical relevance of the cultured A. fumigatus sc isolate was reported (Figure 2).
For 60 A. fumigatus species complex isolates, the resistant phenotype was confirmed in vitro (Table 1). Forty-seven (78.3%) azole-resistant isolates were identified as A. fumigatus sensu strictu. The other 13 azole-resistant isolates were identified as A. lentulus (7 isolates), Neosartorya pseudofisheri (4 isolates), and N. udagawae (2 isolates). Sequence analysis of the cyp51A gene of A. fumigatus showed TR34/L98H in 23 (48.9%) azole-resistant A. fumigatus isolates (Table 2). All TR34/L98H isolates were cultured from patients from European centers. In 3 isolates from the Netherlands, the TR46/Y121F/T289A resistance mechanism was found (7).
A total of 60 azole-resistant isolates were recovered from 46 patients. The overall prevalence of azole resistance among patients with A. fumigatus species complex isolates was 3.2% (range 0.0%–26.1% among the centers). Azole resistance was detected in 11 (57.9%) of 19 participating countries. Acquired resistance in A. fumigatus was found at European sites: Austria, Belgium, Denmark, France, Italy, the Netherlands, Spain, Sweden, and the United Kingdom. In 5 countries (Australia, Germany, Spain, Sweden, and United Kingdom), azole-resistant A. fumigatus sibling species were recovered.
From the 46 patients with resistant isolates, 8 patients had azole-resistant A. fumigatus sibling isolates, and 38 had a resistant A. fumigatus isolate. Of these 38 patients, 19 had an isolate that harbored a fungicide-driven resistance mechanism (i.e., TR34/L98H or TR46/Y121F/T289A). When comparing patients with isolates harboring presumed fungicide-driven resistance mechanisms with “non–fungicide driven” resistance mechanisms (i.e., point mutations or non–Cyp51A-mediated mechanisms), azole exposure differed significantly: 4 (21.1%) of 19 patients with an isolate with the fungicide-driven resistance mechanism had a history of azole therapy, compared with 16 (84.2%) of 19 patients with isolates with other or no cyp51A mutations (p = 0.001). Of the 195 cases with invasive aspergillosis, azole resistance was documented in 10 (5.1%) (3 proven, 1 probable, and 6 possible infections). Among the patients with resistant isolates, 28 patients had documented aspergillus disease (Technical Appendix Table). The case-fatality rate for this cohort was 70%.
Acquired azole resistance in A. fumigatus was detected in 11 of 17 European centers in 9 countries. Overall prevalence of azole resistance was 3.2%; TR34/L98H was the predominant mechanism of resistance (48.9%) in A. fumigatus sensu strictu isolates. This finding substantiates our concern that azole resistance is an emerging problem in A. fumigatus and that resistance selection in the environment contributes significantly to azole-resistant aspergillus diseases. A predilection of isolates harbored the TR34/L98H mutation for patients with acute invasive diseases over patients with aspergilloma and chronic pulmonary aspergillosis. Azole-resistant invasive aspergillosis was documented in 5.1% of cases of invasive aspergillosis, which is not lower than the percentage of the prevalence of azole resistance among the A. fumigatus isolates (3.2%). This finding might indicate that resistance does not come with a significant fitness cost and that azole-resistant isolates that harbored TR34/L98H or TR46/Y121F/T289A are at least as capable of causing invasive aspergillosis as nonresistant wild-type isolates. Although the clinical implications of sibling species of A. fumigatus are less well understood, our study confirms that these species are generally less susceptible than A. fumigatus to azole antifungal drugs.
Our study shows that azole resistance is widespread in Europe. Azole-resistant A. fumigatus caused aspergillus diseases in the patients in our study, and azole resistance was associated with a worsened outcome (3). A rapid and convenient screening method for resistance is indispensable, and centers that care for patients with aspergillus diseases should perform surveillance to determine their local epidemiology. Furthermore, azole resistance has become a public health problem that needs continued international surveillance and research on the mechanisms that enable its selection in the environment. This report of the emergence of resistance has launched a new phase in the management of aspergillus diseases. Unless we can implement measures that prevent the fungicide-driven route of resistance development, the clinical use of azoles will be severely compromised.
Dr. van der Linden is a medical doctor and researcher in the departments of Medical Microbiology and Pediatrics at the Radboud University Medical Centre Nijmegen, Nijmegen, the Netherlands. His research interests include invasive fungal diseases, especially the epidemiology of azole-resistant Aspergillus diseases.
Acknowledgments
We thank Jan Zoll, Ton Rijs, Hein van der Lee, Diane Lamers-Jansen, and Birgit Brandt for excellent technical assistance. We also thank Monica Slavin, Orla Morrissey, and Alison Campbell for local site support.
This work was supported in part by an unrestricted research grant through the Investigator Initiated Studies Program (IISP) of Merck, Sharp & Dohme (MSD).
We declare that we have no conflicts of interest related to this study. J.L. received travel grants from Gilead and MSD and honorarium as a speaker from Pfizer. M.A. has received research grants, travel grants, and honorarium as a speaker or advisor from Astellas, Gilead, MSD, and Pfizer. A.W. received educational grants from Pfizer, Gilead, and MSD. K.L. received research grants from Gilead; Pfizer; and MSD and served on the speakers’ bureau of Pfizer and MSD. S.K. received research funds, travel grants, and honoraria as a speaker or advisor from Gilead, MSD, and Pfizer. E.D. received research grants, travel grants, and honorarium as a speaker or advisor from Astellas, Gilead, MSD, Bio-Rad, Ferrer International, Schering, and Innothera. P.G. received travel grants and honoraria as speaker from Astellas, Gilead, MSD, and Pfizer. J.B. served as a consultant for MSD, Pfizer, Astellas, and Mayne Pharma. C.M. received travel grants from Astellas, honorarium as a speaker from Pfizer, and grant support from Pfizer. C.K. received honoraria from Gilead, MSD, Pfizer, and Astellas. A.U. received a travel grant from Astellas and honoraria as a speaker from MSD and Pfizer. L.N. received travel grants from MSD and Pfizer. P.V. received research grants, has attended conferences, given lectures and participated in advisory boards or trials sponsored by various pharmaceutical companies.
References
- Snelders E, van der Lee HAL, Kuijpers J, Rijs AJ, Varga J, Samson RA, Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5:e219. DOIPubMedGoogle Scholar
- van der Linden JW, Warris A, Verweij PE. Aspergillus species intrinsically resistant to antifungal agents. Med Mycol. 2011;49:S82–9. DOIPubMedGoogle Scholar
- van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, Debets-Ossenkopp YJ, Clinical implications of azole resistance in Aspergillus fumigatus, the Netherlands, 2007–2009. Emerg Infect Dis. 2011;17:1846–54. DOIPubMedGoogle Scholar
- van der Linden JWM, Arendrup MC, van der Lee HAL, Melchers WJG, Verweij PE. Azole containing agar plates as a screening tool for azole resistance of Aspergillus fumigatus. Mycoses. 2009;52: S1–19.9.
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Revised definitions of invasive fungal disease from the EORTC/MSG. Clin Infect Dis. 2008;46:1813–21. DOIPubMedGoogle Scholar
- Lass-Florl C, Cuenca-Estrella M, Denning D, Rodriguez-Tudela J. Antifungal susceptibility testing in Aspergillus spp. according to EUCAST methodology. Med Mycol. 2006;44:319–25. DOIGoogle Scholar
- van der Linden JW, Camps SMT, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Aspergillosis due to voriconazole highly-resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57:513–20. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 21, Number 6—June 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
J.W.M. van der Linden, Departments of Medical Microbiology and Pediatrics, Radboud University Medical Centre Nijmegen, P.O. Box 9101, 6500 HB Nijmegen, the Netherlands
Top