Volume 21, Number 6—June 2015
Letter
MRSA spa t1081, a Highly Transmissible Strain Endemic to Hong Kong, China, in the Netherlands
To the Editor: Control of methicillin-resistant Staphylococcus aureus (MRSA) is an international public health priority. The Netherlands is among countries in Europe that have a low prevalence of MRSA among humans, largely because of a national search and destroy policy (1). The overall prevalence in long-term care facilities (LTCFs) is low (2). However, this policy is challenged by an increase in MRSA S. aureus protein A (spa) t1081, which specifically affects LTCFs. MRSA with the same spa type is endemic to Hong Kong, China, and affects hospitals and LTCFs (3–5). This finding prompted us to jointly explore epidemiologic and strain-related factors.
The low prevalence of MRSA enables the National Institute for Public Health and the Environment (Bilthoven, the Netherlands) to type all first MRSA isolates referred from clinical laboratories in the Netherlands. In 2007, spa typing replaced pulsed-field gel electrophoresis typing. The annual number of referred isolates ranged from 1,570 in 2008 to 2,439 in 2013, excluding livestock-associated strains. Numbers of MRSA spa t1081 isolates were low during 2007–2009 but increased to 127 isolates in 2011 and 218 isolates in 2013.
The search and destroy policy in the Netherlands requires that detection of MRSA infection is followed by screening of neighboring patients and personnel in successive circles until no new colonizations are found. Most reported t1081 isolates represent colonization. In 2013, there were 30 infection isolates, 19 unknown isolates, and 169 colonization isolates.
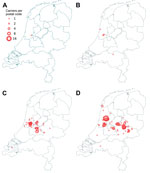
Severe illness caused by t1081 is rarely reported, and eradication therapy is usually successful. In LTCFs, MRSA t1081 was more prevalent, accounting for 27% (65/242) and 24% (72/299) of all MRSA isolates from LTCFs in 2011 and 2013 respectively. LTCF clusters were often small, but some became large. The t1081 strain was probably introduced into Amsterdam and subsequently spread eastward (Figure).
We sequenced 5 t1081 isolates, 3 from The Netherlands (NL2007, NL2011, and NL2013) and 2 from Hong Kong (HK2005 and HK2008), by using whole-genome sequencing (Illumina, San Diego, CA, USA). Sequence data showed the CC45/agr IV-MRSA V type (3,6). A total of 91.39% of reads of NL2007 aligned with the published CC45/USA600 sequence (7); NL2007 was more similar to HK2005 and HK2008 (97.32% and 97.61% identity, respectively). NL2011 and NL2013 showed slightly decreasing similarity to HK2005 (96.86% and 96.59% identity, respectively) and HK2008 (96.97% and 96.69% identity, respectively). Staphylococcal cassette chromosome mec type V sequences in our isolates were more closely related to each other than to the closest reference sequence (GenBank accession no. AB505629), which originated from a CC398 isolate.
Phenotypic resistance to tetracycline and ciprofloxacin is common in t1081 and is often combined with gentamicin and macrolide resistance. tetK, a gene coding resistance to tetracycline that is located on the staphylococcal cassette chromosome mec element, was detected in reads of all sequenced isolates. The macrolide resistance gene ermC on plasmid pKH19 (GenBank accession no. NC_010685.1) was detected in HK2008, NL2011, and NL2013. Resistance to gentamicin (aacA/aphD genes) was detected in all isolates. A recent report on epidemic MRSA strain 15 based on many whole-genome sequenced strains highlights antimicrobial drug use as an evolutionary driving force (8). The tetK gene might benefit t1081 in LTCFs in the Netherlands, in which doxycycline is used more frequently than in hospitals (NethMap-MARAN 2014; http://www.swab.nl/nethmap).
None of our isolates was positive for Panton-Valentine leukocidin, and all isolates had the collagen-binding adhesion gene. In methicillin-sensitive S. aureus, this gene has been associated with carriage (9). The apparent high transmissibility of t1081 remains to be explained.
The present t1081 outbreak has elicited a debate on the policy in the Netherlands. Some elder-care physicians question benefits and costs of this policy for a strain that is weakly pathogenic. Residents in whom MRSA carriage cannot be eradicated face prolonged measures that some physicians say are unethical. Conversely, hospital infection control professionals emphasize that if MRSA can be controlled in hospitals, why not in LTCFs? The search and destroy policy in the Netherlands faced a major challenge in 2001 (10). Uncontrolled dissemination of MRSA had occurred throughout a large hospital in Rotterdam among patients and staff, as well as in neighboring institutions. This outbreak was eventually controlled (10). The Rotterdam area, which is southwest of Amsterdam, has not been affected by the current t1081 outbreak.
Concurrent with this professional debate, a public debate is ongoing on the quality of care in LTCFs. Residents have more illnesses than a decade ago because of increasingly stringent admission criteria. Skill levels of personnel have not kept pace in several LTCFs, as noted by the Health Care Inspectorate. Although virtually all LTCFs are publicly funded, quality differences are substantial. This situation is no longer acceptable, according to public opinion. A link between insufficient skill levels in specific LTCFs and spread of MRSA can be inferred.
The latest initiative to control multidrug-resistant organisms, including MRSA in LTCFs, is included in an existing program for rapid outbreak reporting and support for hospitals by the National Institute for Public Health and the Environment. This initiative is expected to begin early in 2015 and should facilitate control of MRSA in LTCFs.
Acknowledgment
This study was supported by grants from the Health and Medical Research Fund (formerly Research Fund for the Control of Infectious Diseases) of the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region.
References
- Bode LG, Wertheim HF, Kluytmans JA, Bogaers-Hofman D, Vandenbroucke-Grauls CM, Roosendaal R, Sustained low prevalence of meticillin-resistant Staphylococcus aureus upon admission to hospital in the Netherlands. J Hosp Infect. 2011;79:198–201. DOIPubMedGoogle Scholar
- Greenland K, Rijnders MI, Mulders M, Haenen A, Spalburg E, van de Kassteele J, Low prevalence of methicillin-resistant Staphylococcus aureus in Dutch nursing homes. J Am Geriatr Soc. 2011;59:768–9. DOIPubMedGoogle Scholar
- Ho PL, Lai EL, Chow KH, Chow LS, Yuen KY, Yung RW. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in residential care homes for the elderly in Hong Kong. Diagn Microbiol Infect Dis. 2008;61:135–42. DOIPubMedGoogle Scholar
- Cheng VC, Chan JF, Lau EH, Yam WC, Ho SK, Yau MC, Studying the transmission dynamics of meticillin-resistant Staphylococcus aureus in Hong Kong using spa typing. J Hosp Infect. 2011;79:206–10. DOIPubMedGoogle Scholar
- Cheng VC, Tai JW, Wong ZS, Chen JH, Pan KB, Hai Y, Transmission of methicillin-resistant Staphylococcus aureus in the long term care facilities in Hong Kong. BMC Infect Dis. 2013;13:205. DOIPubMedGoogle Scholar
- Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE. 2011;6:e17936. DOIPubMedGoogle Scholar
- Stegger M, Driebe EM, Roe C, Lemmer D, Bowers JR, Engelthaler DM, Genome Sequence of Staphylococcus aureus strain CA-347, a USA600 methicillin-resistant isolate. Genome Announc. 2013;1:e00517–13.
- Holden MT, Hsu LY, Kurt K, Weinert LA, Mather AE, Harris SR, A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res. 2013;23:653–64. DOIPubMedGoogle Scholar
- Rasmussen G, Monecke S, Ehricht R, Soderquist B. Prevalence of clonal complexes and virulence genes among commensal and invasive Staphylococcus aureus isolates in Sweden. PLoS ONE. 2013;8:e77477. DOIPubMedGoogle Scholar
- van Trijp MJ, Melles DC, Hendriks WD, Parlevliet GA, Gommans M, Ott A. Successful control of widespread methicillin-resistant Staphylococcus aureus colonization and infection in a large teaching hospital in the Netherlands. Infect Control Hosp Epidemiol. 2007;28:970–5. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 21, Number 6—June 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Paul Gruteke, Medisch Microbiologisch Laboratorium, Onze Lieve Vrouwe Gasthuis, Oosterpark 9, PO Box 95500, 1090 HM Amsterdam, the Netherlands
Top