Volume 23, Number 1—January 2017
CME ACTIVITY - Research
Analysis of Anthrax Immune Globulin Intravenous with Antimicrobial Treatment in Injection Drug Users, Scotland, 2009–2010
Figure 1
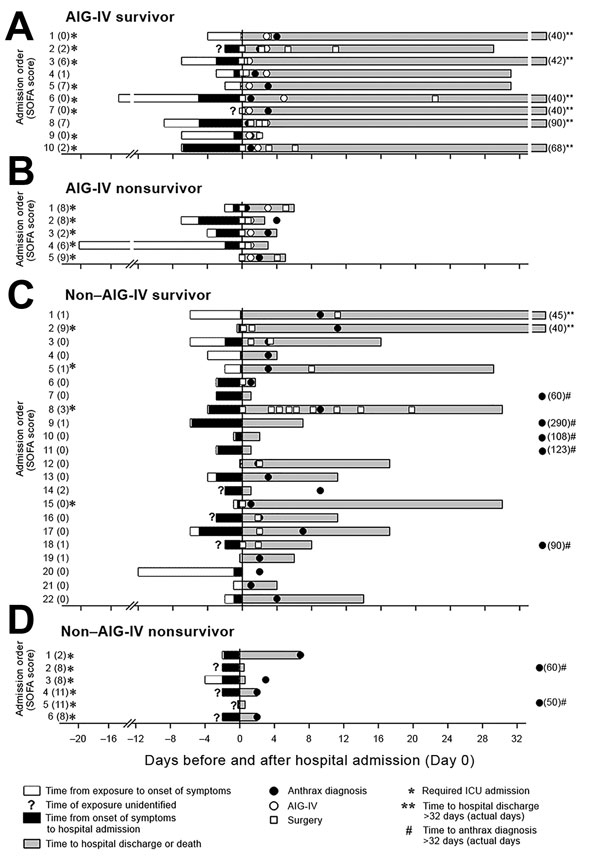
Figure 1. Key events during the illness courses of 15 patients who received AIG-IV (10 survivors, 5 nonsurvivors) and 28 patients who did not receive AIG-IV (22 survivors, 6 nonsurvivors) from the time of their suspected exposure to contaminated heroin to the time of discharge from hospital or to death, Scotland, UK, 2009–2010. A) AIG-IV recipient who survived. B) AIG-IV recipient who died. C) AIG-IV nonrecipient who survived. D) AIG-IV nonrecipient who died. AIG-IV, anthrax immune globulin intravenous; ICU, intensive care unit; SOFA, sequential organ failure.
1These authors contributed equally to this article.
Page created: December 14, 2016
Page updated: December 14, 2016
Page reviewed: December 14, 2016
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.