Volume 26, Number 10—October 2020
Synopsis
Nationwide External Quality Assessment of SARS-CoV-2 Molecular Testing, South Korea
Figure 1
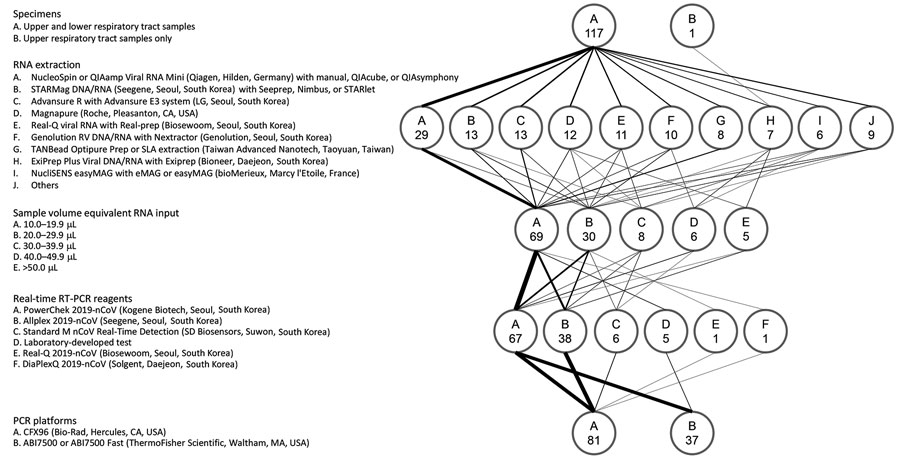
Figure 1. Protocols used for real-time RT-PCR in 118 laboratories participating in an external quality assessment of severe acute respiratory syndrome coronavirus 2 testing, South Korea, March 23–27, 2020. The flow diagram shows the variations in specimens tested, RNA extraction platforms, PCR reagents and amplification platforms, and sample volume equivalent RNA input used in the PCR reaction. The weight of the lines reflects the number of laboratories using a particular step. Numbers in the circles indicate number of laboratories. RT-PCR, reverse transcription PCR.
1These authors contributed equally to this article.
Page created: July 14, 2020
Page updated: September 17, 2020
Page reviewed: September 17, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.