Volume 28, Number 12—December 2022
Research Letter
Human Thelaziosis Caused by Thelazia callipaeda Eyeworm, Hungary
Abstract
Ocular infections with Thelazia callipaeda eyeworms in Europe have become more common. We report a case in Hungary caused by T. callipaeda eyeworms in a 45-year-old woman who had no travel history abroad.
Thelazia spp. (Spirurida, Thelaziidae) are vectorborne zoonotic nematodes that can parasitize conjunctiva and surrounding structures of wild and domestic animals as well as humans (1). Before 2022, a total of 16 species of Thelazia had been described; 3 species, T. callipaeda, T. californiensis, and T. gulosa, are known to infect humans. T. callipaeda nematodes, commonly known as eyeworms, cause autochthonous cases in Europe (2). The earliest reported endemic infection in Europe was detected in a dog in the Piedmont region of Italy in 1989. Since then, several animal and human cases have been documented throughout Europe (Appendix Table 1) (1–4). In Europe, under natural conditions, the only known vector and intermediate host of T. callipaeda eyeworms is the lachryphagous male Phortica variegate fly (1,5). The biologic activity of the fly is affected by temperature (20°C–25°C) and relative humidity (50%–75%) (1,6). The most common clinical manifestations of T. callipaeda infections are lacrimation, foreign body sensation, itchiness, conjunctivitis, and follicular hypertrophy of the conjunctiva; the affected eye may also show severe keratitis and corneal ulceration. Treatment of this infection in humans is primarily the mechanical removal of worms, which is more difficult in their immature stages (7).
In Hungary, T. callipaeda infection has been described in dogs (3). We report a case of conjunctivitis in a human caused by T. callipaeda eyeworms. Our goal is to draw the scientific community’s attention to this spillover event.
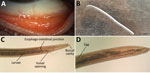
A 45-year-old woman in Hungary was referred to an ophthalmologist in September 2020; she had a foreign-body sensation and redness in her left eye for a week. Slit-lamp examination revealed conjunctivitis. Empiric tobramycin and dexamethasone therapies were initiated. At the time of follow-up, 3 thin, creamy white live worms were removed from the conjunctival fornices of her left eye (Figure). Left eye examination revealed follicular conjunctival hypertrophy. The cornea was not affected, visual acuity was 20/20 on both eyes, and intraocular pressure was in the normal range. The patient’s medical history was uneventful. Laboratory examination showed no elevated leukocytes, C-reactive protein, or erythrocyte sedimentation rate. The patient had no peripheral blood eosinophilia. Because of worsened conjunctivitis, 2% boric acid was applied for 5 days after removal of the worms. On the last follow-up visit, the patient had no symptoms.
Two worms were sent to the Department of Medical Microbiology, University of Szeged (Szeged, Hungary). We examined them under a light microscope (Leica DM 100; Leica Microsystems, https://www.leica-microsystems.com). Wet-mount preparation showed the presence of a characteristic vase-shaped buccal cavity (Figure, panel C) and serrated cuticula with transverse striations. The position of the vulva was anterior to the esophago-junction (8). The anterior half of the abdominal cavity contained first-stage larvae; the posterior part contained eggs. The nematodes were 15 mm and 14 mm long. We identified them as female T. callipaeda eyeworms on the basis of morphologic features. We isolated total DNA from one using QIAamp DNA mini kit (QIAGEN, https://www.qiagen.com) in accordance with tissue protocol; we then performed an in-house PCR targeting cox1 as described previously by Čabanová et al (9). Sequence analysis of the amplified PCR product (GenBank accession no. OP278871) showed 100% homology with T. callipaeda haplotype 1 strains in GenBank (Appendix Figure 1).
We describe an autochthonous case of a patient with T. callipaeda eyeworm ocular infection in Hungary. The patient had no travel history abroad. In July, she visited the Bükk National Park in northeastern Hungary, where she saw a lot of flies. This vector is widely distributed in southern and central Europe and exists in Hungary as well (1,10). Its lachryphagous activity depends mostly on temperature, so climatic changes affect the spread of infection toward the north, affecting new areas (1,6). Until December 2017, a total of 10 canine thelaziosis cases were identified by Farkas et al. in Hungary (3). Most of them visited the same park located in Borsod-Abaúj-Zemplén as our patient (3). That study also suggested that wild carnivores, mainly red foxes, had a role in spreading thelaziosis beyond the border (3). The emergence of human thelaziosis may be explained by the fact that the number of red foxes in Hungary has tripled during the past 50 years (Appendix Figure 2) (Appendix reference 11). In human case-patients, the first-choice therapy is to remove the worms mechanically by flushing the conjunctival sac with sterile physiologic saline under local anesthesia (Appendix reference 12).
From a therapeutic and epidemiologic standpoint, it is important to differentiate between infectious and allergic conjunctivitis. Furthermore, diagnosis can be difficult because immature larvae can hide in the excretory ducts of the lacrimal glands (7). Our findings indicate the need for education and raised awareness about this infection especially for ophthalmologists. Early and adequate diagnosis can help to prevent complications such as corneal ulceration.
Ms. Juhász is a biologist specializing in clinical microbiology in the Department of Medical Microbiology, University of Szeged, Szeged, Hungary. Her primary research interests are human viral, bacterial, and parasitic infections, focusing on respiratory tract pathogens.
References
- Palfreyman J, Graham-Brown J, Caminade C, Gilmore P, Otranto D, Williams DJL. Predicting the distribution of Phortica variegata and potential for Thelazia callipaeda transmission in Europe and the United Kingdom. Parasit Vectors. 2018;11:272. DOIPubMedGoogle Scholar
- do Vale B, Lopes AP, da Conceição Fontes M, Silvestre M, Cardoso L, Coelho AC. Systematic review on infection and disease caused by Thelazia callipaeda in Europe: 2001-2020. Parasite. 2020;27:52. DOIPubMedGoogle Scholar
- Farkas R, Takács N, Gyurkovszky M, Henszelmann N, Kisgergely J, Balka G, et al. The first feline and new canine cases of Thelazia callipaeda (Spirurida: Thelaziidae) infection in Hungary. Parasit Vectors. 2018;11:338. DOIPubMedGoogle Scholar
- Morgado ACT, do Vale B, Ribeiro P, Coutinho T, Santos-Silva S, de Sousa Moreira A, et al. First report of human Thelazia callipaeda infection in Portugal. Acta Trop. 2022;231:
106436 . DOIPubMedGoogle Scholar - Rolbiecki L, Izdebska JN, Franke M, Iliszko L, Fryderyk S. The vector-borne zoonotic nematode Thelazia callipaeda in the eastern part of Europe, with a clinical case report in a dog in Poland. Pathogens. 2021;10:55. DOIPubMedGoogle Scholar
- Pombi M, Marino V, Jaenike J, Graham-Brown J, Bernardini I, Lia RP, et al. Temperature is a common climatic descriptor of lachryphagous activity period in Phortica variegata (Diptera: Drosophilidae) from multiple geographical locations. Parasit Vectors. 2020;13:89. DOIPubMedGoogle Scholar
- do Vale B, Lopes AP, da Conceição Fontes M, Silvestre M, Cardoso L, Coelho AC. Thelaziosis due to Thelazia callipaeda in Europe in the 21st century-A review. Vet Parasitol. 2019;275:
108957 . DOIPubMedGoogle Scholar - Otranto D, Lia RP, Buono V, Traversa D, Giangaspero A. Biology of Thelazia callipaeda (Spirurida, Thelaziidae) eyeworms in naturally infected definitive hosts. Parasitology. 2004;129:627–33. DOIPubMedGoogle Scholar
- Čabanová V, Kocák P, Víchová B, Miterpáková M. First autochthonous cases of canine thelaziosis in Slovakia: a new affected area in Central Europe. Parasit Vectors. 2017;10:179. DOIPubMedGoogle Scholar
- Papp L. Dipterous guilds of small-sized feeding sources in forests of Hungary. Acta Zool Acad Sci Hung. 2002;48:197–213.
Figure
Cite This ArticleOriginal Publication Date: November 15, 2022
Table of Contents – Volume 28, Number 12—December 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Gabriella Terhes, Department of Medical Microbiology, University of Szeged, H-6725 Szeged, Semmelweis street 6, Szeged, Hungary
Top