Volume 29, Number 12—December 2023
Research
Molecular Detection and Characterization of Mycoplasma spp. in Marine Mammals, Brazil
Abstract
Mycoplasma spp. are wall-less bacteria able to infect mammals and are classified as hemotropic (hemoplasma) and nonhemotropic. In aquatic mammals, hemoplasma have been reported in California sea lions (Zalophus californianus) and river dolphins (Inia spp.). We investigated Mycoplasma spp. in blood samples of West Indian manatees (Trichechus manatus), pinnipeds (5 species), and marine cetaceans (18 species) that stranded or were undergoing rehabilitation in Brazil during 2002–2022. We detected Mycoplasma in blood of 18/130 (14.8%) cetaceans and 3/18 (16.6%) pinnipeds. All tested manatees were PCR-negative for Mycoplasma. Our findings indicate that >2 different hemoplasma species are circulating in cetaceans. The sequences from pinnipeds were similar to previously described sequences. We also detected a nonhemotropic Mycoplasma in 2 Franciscana dolphins (Pontoporia blainvillei) that might be associated with microscopic lesions. Because certain hemoplasmas can cause disease and death in immunosuppressed mammals, the bacteria could have conservation implications for already endangered aquatic mammals.
Marine mammals are a diverse polyphyletic group of animals that are anatomically and physiologically adapted to an aquatic lifestyle and depend on marine ecosystems (1). The group includes members of the order Carnivora, such as pinnipeds, sea otters (Enhydra lutris), marine otters (Lontra felina), and polar bears (Ursus maritimus); order Sirenia, which includes manatees and dugongs (Dugong dugon); and the infraorder Cetacea, which comprises dolphins, whales, and porpoises (1,2). Those diverse species are considered ecosystem sentinels (3,4). The coast of Brazil sustains a high diversity of marine mammals, including 44 cetacean, 8 pinniped, and 1 sirenian species (5,6), at least 8 of which are classified into some level of extinction risk (7,8). Anthropogenic threats, such as pollution, climate change, and bycatch, severely effect marine mammal populations; however, infectious diseases also play a role (9). Information on marine mammal diseases in Brazil is limited, particularly in sirenians, and usually focuses on viral infections (10–14).
Mycoplasmas are a group of pleomorphic cell wall–deficient bacteria of the class Mollicutes that are considered the smallest self-replicant prokaryotes (15). Mycoplasmas have been detected in several mammal species, including humans (16–19). Mycoplasma spp. are divided into 2 distinct groups on the basis of infection tropism: nonhemotropic mycoplasmas and hemotropic mycoplasmas (hemoplasmas) (15,20). Nonhemotropic mycoplasmas usually infect epithelial cells of the respiratory and urinary tracts and have been associated with persistent pneumonia, opportunistic nephritis, encephalopathies, autoimmune disease, and reproductive disorder in multiple species (21–23). Hemotropic mycoplasmas can adhere to erythrocyte surfaces and have been linked to acute and chronic anemia, starvation, and even death, especially when they occur along with other pathogens or in immunosuppressed or immature mammals (24). Regardless, most hemoplasma species cause subclinical infections (19,24).
Available studies have focused on domestic animals, but hemoplasmas have attracted the attention of wildlife researchers in the past decade (23). Hemoplasmas have high infection prevalence and genetic diversity, and hemoplasma DNA has been found in several wild mammals (25,26). In addition, occasional reports of interspecies transmission could indicate zoonotic potential (17,27). Despite the potential for zoonoses, few taxa have been described, and little is known about hemoplasma transmission routes and pathogenesis (19).
Among marine mammals, hemoplasmas have only been reported in pinnipeds, and a study of California sea lions (Zalophus californianus) described an infection rate of 12.4% (17/137) (28). We recently detected hemoplasma in 65.6% (32/50) of sampled river dolphins (Inia geoffrensis and I. boliviensis) from the Amazon Basin, indicating that the bacteria are also circulating in fully aquatic mammals (13). Nonhemotropic mycoplasmas have been detected in marine mammals, particularly in pinnipeds, but only a few cases have been reported in cetaceans (29–35). Of note, Mycoplasma phocicerebrale, one of the species isolated from harbor seals (Phoca vitulina) in 1988, is recognized as the likely cause of seal finger disease in humans, which is transmitted by direct contact with infected seals through bites or handling (35). Despite those reports, little information is available on hemotropic mycoplasma in marine cetaceans and sirenians worldwide or on epitheliotropic mycoplasma in cetaceans of the Southern Hemisphere. We surveyed and characterized Mycoplasma spp. in blood samples of marine mammals that were stranded or undergoing rehabilitation in Brazil during 2002–2022.
Samples
All collected samples described herein are stored in the Marine Mammal Blood and Tissue Bank of the Laboratory of Wildlife Comparative Pathology of the University of São Paulo, São Paulo, Brazil. Samples are periodically surveyed for other pathogens depending on necropsy and histopathological reports from sampled mammals.
Cetaceans
We analyzed samples from 130 marine cetaceans that had stranded alive or dead or were bycaught (caught unintentionally) along the northeastern (62/130) or southeastern (68/130) coast of Brazil during 2011–2022. Samples were from animals of the families Delphinidae (n = 67), Pontoporiidae (n = 42), Kogiidae (n = 14), Balaenopteridae (n = 6), and Physeteridae (n = 1), comprising 18 different species (Table 1). Blood samples were collected from the ventral caudal peduncle in live animals or directly from the heart in dead stranded animals, placed in vacutainer tubes with EDTA, and maintained at −80°C until analysis.
Animals that stranded dead or that died during rehabilitation were necropsied according to standard procedures (36). Age class was established according to total body length (6). Selected tissue samples were fixed in 10% formalin at room temperature. An additional set of samples, including lung, spleen, cerebrum, and liver, was frozen at −80°C until analysis.
Pinnipeds
We analyzed blood samples from 18 pinnipeds that stranded alive during 2018–2022 along the northeastern (1/18) or southeastern (17/18) coast of Brazil, including 9 South American fur seals (Arctocephalus australis), 4 subantarctic fur seals (A. tropicalis), 2 Antarctic fur seals (A. gazella), 1 crabeater seal (Lobodon carcinophaga), and 1 southern elephant seal (Mirounga leonina). Blood samples were collected from the caudal gluteal or interdigital veins, placed in vacutainer tubes with EDTA, and maintained at −80°C until analysis.
Sirenians
We analyzed samples of 24 West Indian manatees (Trichechus manatus) undergoing rehabilitation along the northeastern coast of Brazil. Blood samples were collected from the ventral pectoral fin, placed in vacutainer tubes with EDTA, and maintained at −80°C until processing. Of note, those blood samples were previously screened for herpesvirus and adenovirus (14).
Ethics Approvals
This study was approved by the Ethical Committee in Animal of the School of Veterinary Medicine and Animal Sciences, University of São Paulo (process no. 1698290119). The collection and transportation of all samples was approved by the Brazilian Institute of the Environment and Natural Renewable Resources (license no. 67766). The genetic analysis was approved by the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (approval no. A49250C).
Molecular Assays
We extracted total DNA from 172 blood samples by using the DNeasy Blood & Tissue Kit (QIAGEN, https://www.qiagen.com), according to the manufacturer’s instructions. We screened samples for Mycoplasma spp. DNA by using a real time PCR protocol targeting a 384-bp fragment of the 16S rRNA gene that we adapted from a previous study (25). To molecularly characterize the bacteria, we further subjected confirmed positive samples to a nested PCR targeting a 1,100–1,400-bp fragment of the 16S rRNA gene (37) and to a conventional PCR targeting an 800-bp fragment of the 23S rRNA gene (38). In addition, we extracted and tested 8 tissue samples collected from lung, spleen, cerebrum, and liver of a Franciscana dolphin (Pontoporia blainvillei, Pontoporiidae family) that tested positive for nonhemotropic mycoplasma in blood and of its calf, which was found dead alongside the female, to further investigate a potential systemic infection.
We purified positive amplicons by using ExoSAP-IT PCR Product Cleanup (Affymetrix–Thermo Fisher Scientific, https://www.thermofisher.com) and GFX PCR DNA and Gel purification (Global Life Sciences Sigma-Aldrich, https://www.sigmaaldrich.com), and confirmed by direct Sanger sequencing in both directions. We assembled sequence reads in MEGA 7.0 (39) by using ClustalW (http://www.clustal.org/clustal2) alignment and compared sequences with those available in GenBank by searching BLASTn (https://blast.ncbi.nlm.nih.gov). We calculated nucleotide and amino acid genetic distances to the closest sequences on the basis of p-distance, after editing out the primers. Finally, we used MEGA 7.0 to construct nucleotide maximum-likelihood phylogenetic trees with a bootstrap value of 1,000 replicants and a general time-reversible plus invariant site model for 23S gene and Tamura 3 parameter with inversions and gamma distribution model for the 16S gene (329 bp). We selected those evolutionary models by using jMODELTEST 2.1.10 (My Biosoftware, https://mybiosoftware.com). We omitted all bootstrap frequency values <70.
Statistical Analysis
We performed statistical analyses in GraphPad Prism version 5 (GraphPad Software Inc., https://www.graphpad.com) to establish whether hemoplasma could be associated with the any of the following variables: host family, habitat distribution, sampling region, sampling year, age, or sex. We considered p<0.05 statistically significant. We used χ2 test to analyze differences between sexes and Kruskal–Wallis test to analyze the remaining variables. None of the analyzed variables were statistically significant.
Histopathologic Examination
We analyzed available formalin-fixed samples from 2 positive cases of nonhemotropic Mycoplasma, a Franciscana dolphin (identification [ID] number 85) and its calf (ID173). We embedded samples in paraffin wax, processed according to routine procedures of the School of Veterinary Medicine and Animal Sciences of University of São Paulo, sectioned at 5 μm, and stained with hematoxylin and eosin for light microscopic examination.
Molecular Findings
Cetaceans
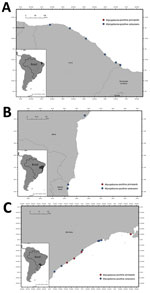
We detected Mycoplasma DNA in the blood of 13.8% (18/130) of tested animals by using the 16S rRNA gene real-time PCR (Figure 1), including 14.9% (10/67) of Delphinidae, 35.7% (5/14) of Kogiidae, and 7.1% (3/42) of Pontoporiidae (Table 1). None of the tested Balaenopteridae and Physeteridae were positive (Table 1). We compiled biologic and molecular data for the Mycoplasma-positive cases (Table 2).
After sequencing the 16S rRNA fragments, we were able to retrieve 1,100-bp sequences from 11 cases and 330-bp sequences from another 5 cases, comprising a total of 12 sequence types. In addition, we confirmed Mycoplasma in 2/18 sequences; however, the low sequence quality prevented species characterization. Among the good quality sequences, 14 Mycoplasma nucleotide sequences from marine dolphins in our study showed >98.0% nt identity with the closest available sequences (GenBank accession nos. ON721294, ON721301, and ON721299), which were previously detected in blood of riverine cetacean species (15). Mycoplasma sequences in our study were from Guiana dolphin (Sotalia guianensis), pygmy sperm whale (Kogia breviceps), dwarf sperm whale (Kogia sima), pygmy killer whale (Feresa attenuata), common dolphin (Delphinus delphis), Franciscana dolphin, killer whale (Orcinus orca), and rough-toothed dolphin (Steno bredanensis). All sequences retrieved from pygmy sperm whales were identical or very similar to sequences described in dwarf sperm whale and had only 2 single point mutations. We also detected a shared 1,100-bp sequence type in samples from a killer whale (ID95), a rough-toothed dolphin (ID81), and a Franciscana dolphin (ID79) that was very similar to the 1 detected in a common dolphin (ID62).
One of the 330-bp sequences detected in Atlantic spotted dolphin (Stenella frontalis) had 92.3% nt identity with an uncultured Mycoplasma spp. (GenBank accession no. OL985926) detected in lowland tapir (Tapirus terrestris) from Brazil (Table 2), likely representing a novel hemoplasma species. Of note, 1 of the retrieved consensus sequences from blood of a Franciscana dolphin had the highest nucleotide identity (98.5%) with a Mycoplasma spp. sequence identified in a fecal sample from Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis) from China that was not classified as hemoplasma. In addition, we detected that nonhemotropic mycoplasma in spleen, liver, and lung of the same dolphin (ID85) and in the cerebrum, spleen, and lung of its calf (ID173).
We were able to recover 23S rRNA Mycoplasma spp. genes from 4/18 16S rRNA real-time PCR–positive cases in a killer whale (1/1), a pygmy sperm whale (1/3), a dwarf sperm whale (1/2), and a pigmy killer whale (1/1) (Table 1). The retrieved 800-bp sequences confirmed 88.4%–90.9% nt identity with a sequence of Candidatus Mycoplasma haemolamae (GenBank accession no. CP003731) detected in alpacas (Vicugna pacos) from the United States (Appendix Figure 1). All but 2 sequences clustered in the 16S rRNA gene phylogram with other sequences from river dolphins within the Candidatus M. haemosuis group (Figure 2, panel A). The sequence retrieved from Atlantic spotted dolphin clustered with other sequences of hemotropic mycoplasma within the M. haemofelis group, and the sequence detected in Franciscana dolphin clustered with the epithelitropic mycoplasma M. pneumoniae (Figure 2, panel A). In the 23S rRNA phylogram, all cetacean species clustered together (Figure 2, panel B).
Pinnipeds
We detected Mycoplasma DNA in 3 of 18 blood samples (Figure 1), comprising 2 subantarctic fur seals and 1 Antarctic fur seal. We were able to recover two 1,100-bp sequences and one 410-bp sequence of the 16S rRNA gene and two 800-bp sequences of the 23S rRNA gene (Table 1). Phylogenetic analysis of the 16S rRNA gene showed that the retrieved sequences had confirmed (99.3%–100% nt) identity with Mycoplasma previously detected in California sea lions (GenBank accession no. GU124613). Both retrieved sequences of the 23S rRNA gene confirmed (99.0% and 99.1% nt) identity with a hemotropic mycoplasma also detected in California sea lions (GenBank accession no. GU905003). On the phylogram, the retrieved sequences clustered with Candidatus M. haemozalophis within the haemosuis group in both genes (Figure 2). We submitted representative sequences to GenBank (accession nos. OR183450, OR184994, OR185401–4, OR193689, OR193690, OR193698–700, OR193703, OR193728, OR193739–45).
Sirenians
We analyzed samples of 24 West Indian manatees. All manatees tested PCR negative for Mycoplasma DNA.
We detected hemoplasma in cetaceans and pinnipeds in Brazil. We observed a 13.8% occurrence rate in tested cetaceans (18/130), which was lower than rates observed in river dolphins (64%; 32/50) but similar to the rates reported in California sea lions (12.4%; 17/137) (13,28). The spatial distribution of the marine cetaceans we studied is wider and less restricted than the previously studied river dolphin populations, which could affect hemoplasma circulation. Furthermore, the sampling method could have influenced the different occurrence rates among marine and riverine cetaceans. In this study, we sampled marine mammals involved in stranding events that were distributed over a broad geographic area and timeframe, and multiple species and groups were represented. Our previous study involved data collected in scientific captures concentrated in a few days and usually involving animals from the same family group and species (13).
Several previous hemoplasma studies targeted the 16S rRNA gene, a highly conserved genetic region, which could affect the identification of novel species because the gene can be undifferentiable between pathogen species (19). Despite targeting this gene, the nucleotide sequence we retrieved from an Atlantic spotted dolphin showed 92.3% nt identity with the closest sequence available, likely corresponding to a novel hemoplasma species. In the phylogram, that novel species did not cluster with the other hemoplasmas described in cetaceans to date, evidencing high divergency. The species also was classified within the M. haemofelis group (Figure 2), which usually is associated with higher pathogenicity. All the remaining hemoplasma sequences we retrieved from cetaceans had >98.0% nt identity with sequences of previously detected hemoplasma in river dolphins and clustered with those sequences. That high similarity among most of the obtained cetacean sequences likely indicates co-evolution or co-divergence of the pathogen and a common ancestry. Nevertheless, the novel sequence detected in an Atlantic spotted dolphin suggests that at least 2 different species of hemotropic mycoplasmas are circulating in cetaceans in the south Atlantic.
We detected the same 16S rRNA sequence type in 3 different species, Franciscana dolphin, killer whale, and rough-toothed dolphin, which belong to 2 different taxonomic families and the samples were collected in different regions of Brazil. That pattern diverged from the pattern we observed in river dolphins, where we could link genetic structure on the basis of host and collection site (13). That finding potentially indicates interspecies transmission of hemoplasma among marine cetaceans because those species are not geographically separated by natural barriers like the river dolphins we studied. We could only amplify the 23S gene in one of the mentioned animals, which precluded a multilocus analysis.
In pinnipeds, 16S and 23S sequences retrieved in subantarctic and Antarctic fur seals were very similar to sequences previously detected in California sea lions (>98.9% nt identity). Considering the geographic and taxonomic differences among those 3 host species, our findings could indicate a low mutation rate within this bacterium. Antarctic and subantarctic fur seals belong to the genus Arctocephalus and both have reproductive colonies near the Antarctic convergence in the Southern Hemisphere, and dispersal to the Northern Hemisphere has not been reported (5). By contrast, California sea lions are of the genus Zalophus, breed in coastal and offshore islands of California (USA) in the Northern Hemisphere, and disperse farther north after breeding season (40). Thus, the species are unlikely to have natural encounters.
We detected a nonhemotropic mycoplasma in blood and available frozen lung, spleen, and liver tissues from a Franciscana dolphin and frozen brain, lung, and spleen tissues from its calf, indicating systemic infection. Nonhemotropic mycoplasmas have already been detected in other marine mammals, including cetaceans, and were mainly associated with pneumonia and septic polyarthritis (29–35). Despite those detections, little information is available about the pathologic signature of these bacteria because the descriptions are usually linked with co-infections, like avian influenza virus or parasites, that could influence the observed lesions (29,31,41,42). In our study, both dolphins had mild to moderate granulocytic pneumonia with exfoliation of macrophages and pneumocytes in alveoli (Appendix Figure 2). Extensive pulmonary lesions, including pneumonia with granulocytes in the alveoli (43,44), have already been described, suggesting that the lesions observed in our study could be associated with mycoplasma infection. Furthermore, the brain of the calf tested positive for Mycoplasma spp. and demonstrated lesions compatible with neuronal suffering (e.g., neuronophagia). Detection of nonhemotropic mycoplasma in brain has been described in multiple mammals, including humans, seals, cattle, and sheep, and has been recorded mainly in nurslings (i.e., human infants and calves) (23), as observed in this investigation. In sheep, evidence of transmammary transmission and development of encephalitis in the infected lambs has been described (23). Despite isolation from the brains of harbor seals in an epizootic mortality episode in the North Sea 30 years ago, the role of mycoplasmas in central nervous system infections has not been clarified (29). Previous reports of nonhemotropic mycoplasmas in cetaceans were all in species distributed in the Northern Hemisphere, and detection was from lungs, nasopharynx, liver, preputium, and atlantooccipital joints (31,33,34).
We did not find statistically significantly higher hemoplasma occurrence in adults than in juveniles or calves, which differs from what we observed in Amazon (Inia geoffrensis) and Bolivian (I. boliviensis) river dolphins (13). Nevertheless, we detected hemoplasma in blood sampled over the years. Thus, endemicity of detected hemoplasmas in those populations cannot yet be determined. As we observed in Amazonian manatees (Trichechus inunguis) (13), all the West Indian manatees tested herein were PCR negative for mycoplasma. Manatees, unlike cetaceans and pinnipeds, are herbivorous and shed different endoparasites than the other 2 groups, which could explain the lack of mycoplasma-positive manatees (45). Nevertheless, most of the tested mammals had previously stranded as neonates or calves; therefore, they would have had little contact with adult manatees that could be a source of infection.
Some of the species selected for this study are threatened and experiencing decreasing population trends. The Franciscana dolphin and the West Indian manatee are currently classified as vulnerable by the International Union for Conservation of Nature’s Red List of Threatened Species (7). Certain hemoplasma species can cause disease and even death in immunosuppressed mammals, so those pathogens could potentially have conservation implications in aquatic mammals. That observation is especially crucial when considering that aquatic mammals are facing diverse anthropogenic and natural threats, such as aquatic pollution, climate change, and anthropization, which are capable of affecting their immune status and increasing disease susceptibility (46).
In conclusion, we detected Mycoplasma DNA in blood samples of marine cetaceans and pinnipeds in Brazil. Our findings indicate that at least 2 divergent species of hemotropic mycoplasma are circulating in cetaceans. In addition, we detected a nonhemotropic mycoplasma in Franciscana dolphins. The Mycoplasma sequences retrieved from pinnipeds were very similar to sequences previously described in California sea lions. Mycoplasmas were not detected in any of the tested sirenians. Our findings demonstrate a wider host range of hemotropic mycoplasma in cetaceans and pinnipeds and expand epitheliotropic mycoplasmas to cetaceans of the Southern Hemisphere, reinforcing the presence of those bacteria in aquatic mammals under natural conditions. The interspecies transmission, zoonotic potential and pathogenicity of all mycoplasmas detected should prompt additional serosurveys to elucidate the range and possible implications of Mycoplasma infections for marine mammals, especially endangered species.
Dr. Duarte-Benvenuto is a veterinarian and a doctorate student at the Laboratory of Wildlife Comparative Pathology in University of São Paulo, São Paulo, Brazil. Her primary research interest is wildlife disease and conservation, especially of aquatic mammals.
Acknowledgments
This study was funded by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES), Brazilian National Council for Scientific and Technological Development (process nos. 304999/2018-0, 165364/2018-1, 141868/2019-8), São Paulo Research Foundation (nos. 2018/20956-0, 2018/25069-7, and 2019/26794-0), and Society of Marine Mammalogy. C.S. is funded by a fellowship from Juan de la Cierva incorporación (no. IJC2020–046019-I) and I.S. is funded by a fellowship from Juan de la Cierva formación (no. FJC2020–046311–I), which are granted by Agencia Estatal de Investigación, Madrid, Spain. J.L.C.-D. receives a professorship from CNPQ (304106/2022-4).
Marine mammals that stranded along the São Paulo coast were rescued by the Santos Basin Beach Monitoring Project (PMP-BS/ABIO 640/215). PMP-BS is a project required by the Brazilian Institute of the Environment and Renewable Natural Resources of the Brazilian Ministry of Environment for the environmental licensing process of the oil and natural gas production and transport by Petrobras at the Santos Basin. The objective of this project is to assess the possible impacts of oil production and flow activities on birds, sea turtles, and marine mammals, through monitoring of beaches and veterinary care for live animals and necropsy of animals found dead. The project is carried out from Laguna, in Santa Catarina, to Saquarema, in Rio de Janeiro, and is divided into 15 sections. Instituto de Pesquisas Cananéia monitors the Section 7, comprising Ilha do Cardoso, Ilha Comprida, and Juréia (Iguape), Biopesca institute monitors the Section 8, between Peruíbe and Praia Grande, and Argonauta para Conservação Marinha e Costeira institute monitors the Section 10, between São Sebastião and Ubatuba.
References
- Berta A, Sumich JL, Kovacs KM. Marine mammals: evolutionary biology, 2nd edition. London: Elsevier; 2005.
- Society for Marine Mammalogy. List of marine mammal species and subspecies, 2023 [cited 2023 Jun 20]. https://marinemammalscience.org/science-and-publications/list-marine-mammal-species-subspecies
- Bossart GD. Marine mammals as sentinel species for oceans and human health. Vet Pathol. 2011;48:676–90. DOIPubMedGoogle Scholar
- Frainer G, Heissler VL, Moreno IB. A wandering Weddell seal (Leptonychotes weddellii) at Trindade Island, Brazil: the extreme sighting of a circumpolar species. Polar Biol. 2018;41:579–82. DOIGoogle Scholar
- Monteiro-Filho E, De Oliveira L, Monteiro K, Filla G, Quito L, Ferro De Godoy D; Cananéia Research Institute. Illustrated guide to marine mammals of Brazil [in Portuguese] [cited 2023 Apr 14]. https://ipecpesquisas.org.br/wp-content/uploads/2021/03/Guia-Ilustrado-2021-3_interativo_reduzido.pdf
- International Union for Conservation of Nature and Natural Resources. 2021 IUCN red list of threatened species [cited 2021 Jun 18]. https://www.iucnredlist.org
- Chico Mendes Institute for Biodiversity Conservation. National list of endangered species 2022 [in Portuguese] [cited 2023 Jun 26]. https://www.icmbio.gov.br/cepsul/images/stories/legislacao/Portaria/2020/P_mma_148_2022_altera_anexos_P_mma_443_444_445_2014_atualiza_especies_ameacadas_extincao
- Smith KF, Acevedo-Whitehouse K, Pedersen AB. The role of infectious diseases in biological conservation. Anim Conserv. 2009;12:1–12. DOIGoogle Scholar
- Groch KR, Santos-Neto EB, Díaz-Delgado J, Ikeda JMP, Carvalho RR, Oliveira RB, et al. Guiana dolphin unusual mortality event and link to cetacean morbillivirus, Brazil. Emerg Infect Dis. 2018;24:1349–54. DOIPubMedGoogle Scholar
- Sacristán C, Esperón F, Ewbank AC, Costa-Silva S, Marigo J, Matushima ER, et al. Identification of novel gammaherpesviruses in a South American fur seal (Arctocephalus australis) with ulcerative skin lesions. J Wildl Dis. 2018;54:592–6. DOIPubMedGoogle Scholar
- Sacristán C, Esperón F, Ewbank AC, Díaz-Delgado J, Ferreira-Machado E, Costa-Silva S, et al. Novel herpesviruses in riverine and marine cetaceans from South America. Acta Trop. 2019;190:220–7. DOIPubMedGoogle Scholar
- Duarte-Benvenuto A, Sacristán C, Ewbank AC, Sacristán I, Zamana-Ramblas R, Gravena W, et al. Hemotropic Mycoplasma spp. in aquatic mammals, Amazon Basin, Brazil. Emerg Infect Dis. 2022;28:2556–9. DOIPubMedGoogle Scholar
- Ewbank AC, Duarte-Benvenuto A, Zamana-Ramblas R, Sacristán I, Costa-Silva S, Carvalho VL, et al. Herpesvirus and adenovirus surveillance in threatened wild West Indian (Trichechus manatus) and Amazonian manatees (Trichechus inunguis), Brazil. Acta Trop. 2023;237:
106740 . DOIPubMedGoogle Scholar - Brown DR, May M, Bradbury JM, Balish MF, Calcutt MJ, Glass JI, et al. Mycoplasma. In: Whitman WB, DeVos P, Dedysh S, Hedlund B, Kämpfer P, Rainey F, et al. editors. Bergey’s manual of systematics of archaea and bacteria. New York: Springer Science+Business Media; 2015. p. 1–78.
- Pitcher DG, Nicholas RAJ. Mycoplasma host specificity: fact or fiction? Vet J. 2005;170:300–6. DOIPubMedGoogle Scholar
- dos Santos AP, dos Santos RP, Biondo AW, Dora JM, Goldani LZ, de Oliveira ST, et al. Hemoplasma infection in HIV-positive patient, Brazil. Emerg Infect Dis. 2008;14:1922–4. DOIPubMedGoogle Scholar
- Kumar A, Rahal A, Chakraborty S, Verma AK, Dhama K. Mycoplasma agalactiae, an etiological agent of contagious agalactia in small ruminants: a review. Vet Med Int. 2014;2014:
286752 . DOIPubMedGoogle Scholar - Millán J, Di Cataldo S, Volokhov DV, Becker DJ. Worldwide occurrence of haemoplasmas in wildlife: Insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound Emerg Dis. 2021;68:3236–56. DOIPubMedGoogle Scholar
- Willi B, Boretti FS, Tasker S, Meli ML, Wengi N, Reusch CE, et al. From Haemobartonella to hemoplasma: molecular methods provide new insights. [b]. Vet Microbiol. 2007;125:197–209. DOIPubMedGoogle Scholar
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–156. DOIPubMedGoogle Scholar
- Hitti J, Garcia P, Totten P, Paul K, Astete S, Holmes KK. Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex Transm Dis. 2010;37:81–5. DOIPubMedGoogle Scholar
- Rosales RS, Puleio R, Loria GR, Catania S, Nicholas RAJ. Mycoplasmas: Brain invaders? Res Vet Sci. 2017;113:56–61. DOIPubMedGoogle Scholar
- Sykes JE, Tasker S. Hemoplasma infections. In: Sykes J, editor. Canine and feline infectious diseases. St. Louis: Saunders (Elsevier): 2015. p. 390–8.
- Cabello J, Altet L, Napolitano C, Sastre N, Hidalgo E, Dávila JA, et al. Survey of infectious agents in the endangered Darwin’s fox (Lycalopex fulvipes): high prevalence and diversity of hemotrophic mycoplasmas. Vet Microbiol. 2013;167:448–54. DOIPubMedGoogle Scholar
- Di Cataldo S, Hidalgo-Hermoso E, Sacristán I, Cevidanes A, Napolitano C, Hernández CV, et al. Hemoplasmas are endemic and cause asymptomatic infection in the endangered Darwin’s fox (Lycalopex fulvipes). Appl Environ Microbiol. 2020;86:e00779–20. DOIPubMedGoogle Scholar
- Maggi RG, Compton SM, Trull CL, Mascarelli PE, Mozayeni BR, Breitschwerdt EB. Infection with hemotropic Mycoplasma species in patients with or without extensive arthropod or animal contact. J Clin Microbiol. 2013;51:3237–41. DOIPubMedGoogle Scholar
- Volokhov DV, Norris T, Rios C, Davidson MK, Messick JB, Gulland FM, et al. Novel hemotrophic mycoplasma identified in naturally infected California sea lions (Zalophus californianus). Vet Microbiol. 2011;149:262–8. DOIPubMedGoogle Scholar
- Giebel J, Meier J, Binder A, Flossdorf J, Poveda JB, Schmidt R, et al. Mycoplasma phocarhinis sp. nov. and Mycoplasma phocacerebrale sp. nov., two new species from harbor seals (Phoca vitulina L.). Int J Syst Bacteriol. 1991;41:39–44. DOIPubMedGoogle Scholar
- Ruhnke HL, Madoff S. Mycoplasma phocidae sp. nov., isolated from harbor seals (Phoca vitulina L.). Int J Syst Bacteriol. 1992;42:211–4. DOIPubMedGoogle Scholar
- Foster G, McAuliffe L, Dagleish MP, Barley J, Howie F, Nicholas RAJ, et al. Mycoplasma species isolated from harbor porpoises (Phocoena phocoena) and a Sowerby’s beaked whale (Mesoplodon bidens) stranded in Scottish waters. J Wildl Dis. 2011;47:206–11. DOIPubMedGoogle Scholar
- Lynch M, Taylor TK, Duignan PJ, Swingler J, Marenda M, Arnould JP, et al. Mycoplasmas in Australian fur seals (Arctocephalus pusillus doriferus): identification and association with abortion. J Vet Diagn Invest. 2011;23:1123–30. DOIPubMedGoogle Scholar
- Díaz-Delgado J, Fernández A, Sierra E, Sacchini S, Andrada M, Vela AI, et al. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006-2012). PLoS One. 2018;13:
e0204444 . DOIPubMedGoogle Scholar - Marón CF, Kohl KD, Chirife A, Di Martino M, Fons MP, Navarro MA, et al. Symbiotic microbes and potential pathogens in the intestine of dead southern right whale (Eubalaena australis) calves. Anaerobe. 2019;57:107–14. DOIPubMedGoogle Scholar
- White CP, Jewer DD. Seal finger: A case report and review of the literature. Can J Plast Surg. 2009;17:133–5. DOIPubMedGoogle Scholar
- Geraci JR, Lounsbury VJ. Marine mammals ashore: a field guide for strandings, 2nd ed. College Station (TX): Texas A&M University; 2005.
- Harasawa R, Orusa R, Giangaspero M. Molecular evidence for hemotropic Mycoplasma infection in a Japanese badger (Meles meles anakuma) and a raccoon dog (Nyctereutes procyonoides viverrinus). J Wildl Dis. 2014;50:412–5. DOIPubMedGoogle Scholar
- Mongruel ACB, Spanhol VC, Valente JDM, Porto PP, Ogawa L, Otomura FH, et al. Survey of vector-borne and nematode parasites involved in the etiology of anemic syndrome in sheep from Southern Brazil. Rev Bras Parasitol Vet. 2020;29:
e007320 . DOIPubMedGoogle Scholar - Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. DOIPubMedGoogle Scholar
- Carretta JV, Forney KA, Muto MM, Barlow J, Baker J, Hanson B, et al. Pacific marine mammal stock assessments, technical memorandum, NOAA-TM-NMFS-SWSC. Washington: National Oceanic and Atmospheric Administration; 2006.
- Haulena M, Gulland FM, Lawrence JA, Fauquier DA, Jang S, Aldridge B, et al. Lesions associated with a novel Mycoplasma sp. in California sea lions (Zalophus californianus) undergoing rehabilitation. J Wildl Dis. 2006;42:40–5. DOIPubMedGoogle Scholar
- Berhane Y, Joseph T, Lung O, Embury-Hyatt C, Xu W, Cottrell P, et al. Isolation and characterization of novel reassortant influenza A(H10N7) virus in a harbor seal, British Columbia, Canada. Emerg Infect Dis. 2022;28:1480–4. DOIPubMedGoogle Scholar
- Bölske G, Engvall A, Renström LH, Wierup M. Experimental infections of goats with Mycoplasma mycoides subspecies mycoides, LC type. Res Vet Sci. 1989;46:247–52. DOIPubMedGoogle Scholar
- Dawood A, Algharib SA, Zhao G, Zhu T, Qi M, Delai K, et al. Mycoplasmas as host pantropic and specific pathogens: clinical implications, gene transfer, virulence factors, and future perspectives. Front Cell Infect Microbiol. 2022;12:
855731 . DOIPubMedGoogle Scholar - Campbell HW, Irvine AB. Feeding ecology of the West Indian manatee Trichechus manatus Linnaeus. Aquaculture. 1977;12:249–51. DOIGoogle Scholar
- Sanderson CE, Alexander KA. Unchartered waters: Climate change likely to intensify infectious disease outbreaks causing mass mortality events in marine mammals. Glob Change Biol. 2020;26:4284–301. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: November 06, 2023
Table of Contents – Volume 29, Number 12—December 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Aricia Duarte-Benvenuto, Laboratório de Patologia Experimental e Comparada, 87 Prof. Dr. Orlando Marques de Paiva Ave, São Paulo 05508270, Brazil
Top