Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 30, Number 10—October 2024
Dispatch
Bluetongue Virus in the Iberian Lynx (Lynx pardinus), 2010–2022
Suggested citation for this article
Abstract
Clinical infection and death caused by bluetongue virus infection has been reported in the Eurasian lynx. Bluetongue virus surveillance in the Iberian lynx revealed widespread and repeated exposure to serotypes 1 and 4 in wild and captive populations of this species. This exposure is possibly from a spillover event from sympatric ruminants.
Bluetongue virus (BTV) is a reemerging, multihost orbivirus of animal health concern. Although the disease is subject to eradication in some regions of Europe, BTV continues to circulate and spread to new areas of the continent (1,2). Wild ruminants play a role in the continued presence and dissemination of BTV in Iberian ecosystems (3), where they share habitats and natural resources with other wildlife species such as the Iberian lynx (Lynx pardinus). The Iberian lynx, endemic to the Iberian Peninsula, is one of the most vulnerable felids in the world (4). In recent decades, shared pathogens with sympatric species, including ruminants, have caused clinical disease and death in the Iberian lynx (5). Although exposure, clinical infection, and death because of BTV infection have been reported in the Eurasian lynx (L. lynx) (6,7), the susceptibility of Iberian lynxes to BTV is unknown. The objectives of our research were to determine BTV exposure in Iberian lynx populations, to assess potential risk factors associated with viral exposure in this species, and to evaluate the dynamics of seropositivity to BTV in longitudinally sampled animals.
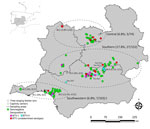
We collected blood, serum, and spleen samples from 340 Iberian lynxes (229 free-ranging and 111 captive) throughout the Iberian Peninsula during 2010–2022 (Table; Figure 1). In addition, longitudinal surveillance was conducted on 50 of the 340 Iberian lynxes during the study period.
We tested serum samples for BTV antibodies by using a double recognition ELISA (Gold Standard Diagnostics, https://www.goldstandarddiagnostics.com). Whenever possible, we tested ELISA-positive serum by using a virus neutralization test (VNT) to detect specific antibodies against BTV-1 and BTV-4 serotypes (Appendix). We assessed the presence of viral RNA in blood and spleen samples from ELISA-seropositive lynxes with available samples (n = 30) and a subset of randomly selected seronegative animals (n = 69) by molecular analyses (Appendix) (8–10). We analyzed associations between the seropositivity to BTV detected by ELISA and explanatory variables by using Pearson χ2 or Fisher exact tests, considering differences significant at p<0.05.
ELISA testing found that 39 (11.5%; 95% CI 8.5–15.3) of the 340 Iberian lynxes were positive for BTV antibodies (Table; Figure 1). We detected significant differences in the ages, sexes, life condition, and sampling region of the animals (Table). Of the 50 longitudinally sampled animals, 4 were seropositive at all samplings, whereas seroconversions were found in 9 animals and seroreversions in 3 animals (Appendix Table 1).
Of the ELISA-seropositive samples, 7 could not be analyzed by using VNT because of cytotoxicity or insufficient sample volume, and 13 of the ELISA-seropositive samples were negative by using VNT (Appendix Table 2). Of the Iberian lynxes analyzed, 19 (59.4%) had antibodies against BTV by both ELISA and VNT. Specific antibodies against serotype BTV-1 were detected in 10 samples (3.0%, 95% CI 1.6%–5.4%) and against BTV-4 were detected in 9 samples (2.7%, 95% CI 1.4%–5.1%). Of note, 2/10 animals with anti-BTV-1 also showed neutralizing activity to BTV-4. Despite having 4-fold lower titers, co-exposure to both serotypes in these animals cannot be ruled out. BTV RNA was detected in the blood of 1 Iberian lynx (1.0%, 95% CI 0.2%–5.5%), which also had specific neutralizing antibodies against BTV-4. Phylogenetic analysis of segments 2, 5, and 10 confirmed the presence of BTV-4 RNA (GenBank accession nos. PP319976–319978) and showed high similarity (>99%) with BTV-4 sequences obtained during the study period from cattle, goats, sheep, and Iberian ibexes in southern Spain (Figure 2).
Our study confirms the exposure of Iberian lynx populations to BTV, increasing the number of wildlife species susceptible to this virus. Although BTV was thought to be transmitted to carnivores through Culicoides biting midges, BTV infection in carnivores appears to occur primarily through the ingestion of infected animals (11). Because of this transmission, the higher seropositivity found in free-ranging animals (14.0%) compared with captive animals (6.3%) might be explained by the trophic behavior of free-ranging Iberian lynxes on wild ungulates (12), which is further supported by the high homology (>99%) of the BTV-4 sequence with those found in wild ungulates from Spain. However, it is also possible that free-ranging lynxes are more frequently exposed to Culicoides because of their larger home range and the lack of periodic cleaning and disinfestation procedures used in captivity centers (13), which could interfere with the breeding cycle of Culicoides.
The higher seroprevalence detected in male compared with female animals aligns with findings in wild ruminants, where male animals’ larger home ranges and body mass increase their risk for contact with Culicoides midges (14). The higher frequency of seropositivity detected in adults (20.6%) compared with subadults (6.0%) and yearlings (4.5%) might be associated with a higher likelihood of cumulative exposure to BTV and lifelong persistence of antibodies in Iberian lynxes (3). Of note, 1 yearling sampled in 2013, 3 yearlings sampled in 2021, and 1 yearling sampled in 2022 were positive for BTV antibodies, suggesting recent exposure to BTV in Iberian lynx populations during these years. This hypothesis is supported by concurrent outbreaks of BTV infection in livestock (15). Furthermore, the 4 BTV-1 seroconversions overlap spatiotemporally with outbreaks of this serotype reported in livestock (15). This finding, together with the higher seroprevalence observed in lynxes from the southern region, which is consistent with the higher number of BTV outbreaks reported in livestock in this area (15), suggests a common epidemiologic pattern of BTV in domestic and wild species. However, 2/5 seropositive yearlings were sampled in the provinces of Jaen and Toledo (southern and central Spain) in February 2013 and January 2021, whereas the previous BTV outbreaks reported in livestock in these provinces occurred in 2010 and 2014 (15). Those findings are consistent with previous reports of BTV circulating in wild ruminants in the absence of BTV outbreaks in livestock in Spain (3) and highlight the importance of wildlife in the epidemiology of this virus in the study area, especially in areas where livestock vaccination has been implemented.
We confirmed the presence of BTV-1 and BTV-4 serotype neutralizing antibodies in both free-ranging and captive Iberian lynxes. This result is consistent with the BTV outbreaks detected in livestock in Spain during the study period, where cases of BTV-1 and BTV-4 were reported (15). The detection of BTV-4 RNA in an Iberian lynx confirms the susceptibility of the species to BTV infection. The infected lynx was a BTV-4–seropositive, free-ranging lynx sampled in southern Spain (municipality of Marmolejo) on November 31, 2021. Exposure to BTV was also detected in a yearling sampled close to this municipality on December 3, 2021, and outbreaks of BTV-4 serotype were reported in domestic ruminants close to this location during this period (15). This finding, together with the high similarity with other sequences obtained from domestic and wild ruminants in Spain, provides evidence that the exposure of Iberian lynxes to BTV could be the result of spillover from domestic or wild sympatric ruminants, indicating a common epidemiologic cycle of BTV among ruminants and Iberian lynxes.
In summary, we report evidence of widespread and repeated exposure to BTV in Iberian lynx populations. Further studies are needed to determine the effects of BTV on the health of Iberian lynx populations, but the low prevalence of infection suggests that this species may be considered a spillover host, rather than a true reservoir of BTV.
Dr. Caballero-Gómez is a postdoctoral researcher at the Maimonides Biomedical Research Institute of Cordoba. His research interests include the epidemiology and diagnosis of emerging and reemerging diseases of public and animal health concerns.
Acknowledgments
We thank the regional governments of Junta de Andalucía and Junta de Castilla-La Mancha for allowing us to carry out this study. We thank the conservation programs of the Iberian lynx. We thank the Laboratorio Regional Agroalimentario y Ambiental de Castilla-La Mancha, especially Sonia Lázaro, for their support.
This study is part of the TED2021-132599B-C22 project, funded by MCIN/AEI/10.13039/501100011033 and the Recovery, Transformation and Resilience Plan, funded by the European Union (Next Generation EU). J.C-G. was supported by the CIBER-Consorcio Centro de Investigación Biomédica en Red-(CB21/13/00083), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and the European Union (Next Generation EU). M.G. was supported by a postdoctoral contract Margarita Salas (University of Murcia) from the Program of Requalification of the Spanish University System (Spanish Ministry of Universities) financed by the European Union-NextGenerationEU. M.S-S. was supported by Investigo Program, funded by European Union (Next Generation EU).
References
- European Commission. About bluetongue. 2023 [cited 2024 Jan 22]. https://food.ec.europa.eu/animals/animal-diseases/surveillance-eradication-programmes-and-disease-free-status/bluetongue_en
- World Animal Health Information System. 2023 [cited 2024 Jan 20]. https://www.woah.org/es/que-hacemos/sanidad-y-bienestar-animal/recopilacion-de-datos-sobre-enfermedades/sistema-mundial-de-informacion-sanitaria
- García-Bocanegra I, Arenas-Montes A, Lorca-Oró C, Pujols J, González MÁ, Napp S, et al. Role of wild ruminants in the epidemiology of bluetongue virus serotypes 1, 4 and 8 in Spain. Vet Res. 2011;42:88. DOIPubMedGoogle Scholar
- International Union for Conservation of Nature. The IUCN red list of threatened species. 2023 [cited 2024 Feb 10]. https://www.iucnredlist.org
- Millán J, Candela MG, Palomares F, Cubero MJ, Rodríguez A, Barral M, et al. Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet J. 2009;182:114–24. DOIPubMedGoogle Scholar
- Jauniaux TP, De Clercq KE, Cassart DE, Kennedy S, Vandenbussche FE, Vandemeulebroucke EL, et al. Bluetongue in Eurasian lynx. Emerg Infect Dis. 2008;14:1496–8. DOIPubMedGoogle Scholar
- Caballero-Gómez J, Cano Terriza D, Pujols J, Martínez-Nevado E, Carbonell MD, Guerra R, et al. Monitoring of bluetongue virus in zoo animals in Spain, 2007-2019. Transbound Emerg Dis. 2022;69:1739–47. DOIPubMedGoogle Scholar
- Toussaint JF, Sailleau C, Breard E, Zientara S, De Clercq K. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J Virol Methods. 2007;140:115–23. DOIPubMedGoogle Scholar
- Gómez-Guillamón F, Caballero-Gómez J, Agüero M, Camacho-Sillero L, Risalde MA, Zorrilla I, et al. Re-emergence of bluetongue virus serotype 4 in Iberian ibex (Capra pyrenaica) and sympatric livestock in Spain, 2018-2019. Transbound Emerg Dis. 2021;68:458–66. DOIPubMedGoogle Scholar
- Hofmann MA, Renzullo S, Mader M, Chaignat V, Worwa G, Thuer B. Genetic characterization of toggenburg orbivirus, a new bluetongue virus, from goats, Switzerland. Emerg Infect Dis. 2008;14:1855–61. DOIPubMedGoogle Scholar
- More S, Bicout D, Bøtner A, Butterworth A, Depner K, Edwards S, et al.; EFSA Panel on Animal Health and Welfare (AHAW). Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): bluetongue. EFSA J. 2017;15:
e04957 .PubMedGoogle Scholar - Gonzálvez M, Jiménez-Ruiz S, Paniagua J, Rouco C, García-Bocanegra I. Vulture feeding stations threaten Iberian lynx. Biol Conserv. 2023;281:
109960 . DOIGoogle Scholar - Rivas A, Boixader J, Vargas A, Pérez MJ, Serra R, Asensio V, et al. Manual del manejo del lince ibérico en cautividad. Programa de conservación ex‐situ del Lin ce Ibérico. 2016 [cited 2024 Jan 22] https://www.lynxexsitu.es/ficheros/documentos_pdf/84/Manual_Manejo_Lince_Iberico_2016.pdf
- Ruiz-Fons F, Sánchez-Matamoros A, Gortázar C, Sánchez-Vizcaíno JM. The role of wildlife in bluetongue virus maintenance in Europe: lessons learned after the natural infection in Spain. Virus Res. 2014;182:50–8. DOIPubMedGoogle Scholar
- Red de Alerta SV. Ministerio de agricultura pesca y alimentación. 2023 [cited 2024 Feb 10]. https://servicio.mapama.gob.es/rasve/Acceso.aspx
Figures
Table
Suggested citation for this article: Caballero-Gómez J, Sánchez-Sánchez M, Lorca-Oró C, Fernández de Mera IG, Zorrilla I, López G, et al. Bluetongue virus in the Iberian lynx (Lynx pardinus), 2010–2022. Emerg Infect Dis. 2024 October [date cited]. https://doi.org/10.3201/eid3010.240235
Original Publication Date: September 18, 2024
Table of Contents – Volume 30, Number 10—October 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Moisés Gonzálvez, Department of Animal Health, Road Madrid-Cadiz Km 396, University of Cordoba, 14014 Cordoba, Spain
Top