Volume 30, Number 7—July 2024
Dispatch
Orthohantaviruses in Misiones Province, Northeastern Argentina
Abstract
Few cases of hantavirus pulmonary syndrome have been reported in northeastern Argentina. However, neighboring areas show a higher incidence, suggesting underreporting. We evaluated the presence of antibodies against orthohantavirus in small rodents throughout Misiones province. Infected Akodon affinis montensis and Oligoryzomys nigripes native rodents were found in protected areas of Misiones.
Orthohantavirus is a genus of globally distributed RNA viruses in the family Hantaviridae. In the Americas, the viruses are hosted by native rodent species within the Cricetidae family (1). Although not all orthohantaviruses cause disease in humans, some genotypes are etiologic agents of hantavirus cardiopulmonary syndrome (HCPS), a serious emerging disease (1). Along with Brazil and Chile, Argentina is among the countries in South America with the highest incidence of HCPS (1). HCPS cases in the country are distributed in 4 epidemiologic regions; incidence is lowest in the northeast. HCPS cases in that region were first registered in 2003 in the south of Misiones province (2). Those cases led to the identification of Lechiguanas virus and Juquitiba virus, 2 Orthohantavirus andesense–like genotypes, as etiologic agents, and of Oligoryzomys nigripes (black-footed pygmy rice rats) as a reservoir of Juquitiba virus, whereas the host of Lechiguanas virus (presumably O. flavescens yellow pygmy rice rats) was not confirmed (2). Almost 15 years later, infected montane grass mice (Akodon montensis) were detected in north Misiones, implicating this species as a new reservoir for orthohantavirus in Argentina (3).
Since 2003, <10 orthohantavirus cases have been diagnosed in Misiones (4,5). However, the circulation of >1 pathogenic genotype and the presence of 3 known orthohantavirus reservoirs, together with a higher incidence of human cases in neighboring states of Brazil, suggest that HCPS might be underreported in this province (2,3,6). Underreporting is likely a result of the high rates of poverty, rurality, and lack of access to healthcare in Misiones (7,8), factors that are known to contribute to underreporting of diseases (9). To identify areas with a potential for higher risk for HCPS, identifying areas where pathogenic orthohantavirus circulates within the rodent community is crucial. In this study, we sought to estimate the seroprevalence of orthohantavirus (as a proxy for infection) and identify the main hosts in protected areas of Misiones. This research was reviewed and approved by the institutional animal care and use committee of the University of Buenos Aires (Faculty of Natural and Exact Sciences).
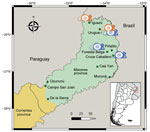
We conducted 24 trapping sessions spanning 2–4 consecutive nights in 10 protected areas throughout Misiones Province: Iguazú National Park and Urugua-í Provincial Park in the north; Cruce Caballero, Piñalito, Caá Yarí, and Moconá provincial parks and Forestal Belga protected area in the central part of the province; and Osununú Natural Reserve, Campo San Juan Federal Park, and De las Sierras Provincial Park in the south (Figure 1). We live-trapped rodents during October 2019–February 2023. In each area, we set 60–200 Sherman traps, plus 90 cage traps in some areas, along tracks in the woods. We baited Sherman traps using a mixture of peanut butter, fat, and rolled oats (plus bananas and sardines in most trapping sessions), whereas we baited cage traps with chicken meat and carrots. We identified captured animals up to the last taxonomic level possible according to external morphology. We recorded sex and reproductive conditions of individual rodents. We obtained a blood sample from a small cut on the tip of the tail and later used that sample to analyze the presence of antibodies against orthohantaviruses by using ELISA (10). To estimate the diversity of the small rodent community in each study area, we calculated richness, Shannon-Wiener diversity index (−Σpi × ln (pi), where pi is the relative proportion of species i in the community), evenness (H/Hmax, where Hmax = ln[S]), and Simpson diversity index (1 − Σpi2) using the overall data per trapping area.
A total of 12,424 trap nights yielded 765 rodents of 9 species, resulting in an overall trap success of 6.16%. Species consisted of A. affinis montensis mice (656), Oligoryzomys sp. rodents (28, 1 confirmed as O. nigripes), Sooretamys angouya (rat-headed rice rat) (23), Thaptomys nigrita (blackish grass mouse) (17), Nectomys squamipes (scaly-footed water rat) (14), Euryoryzomys russatus (big-headed rice rat) (11), Brucepattersonius iheringi (Ihering’s akodont) (9), Oxymycterus quaestor (quaestor hocicudo) (2), and Rattus rattus (black rat) (5) (Table 1).
We detected antibodies against orthohantavirus in A. aff. montensis mice with an overall seroprevalence of 0.007; overall seroprevalence in Oligoryzomys sp. rodents was 0.083 (Table 2). Seropositive rodents were captured in 4 natural areas, Urugua-í, Cruce Caballero, Iguazú, and Forestal Belga; Urugua-í was the only area in which antibodies were found in both species (Table 2; Figure 1). Because of its relevance to this research, the seropositive Oligoryzomys sp. rodent captured in Iguazú National Park was identified at the species level through molecular characterization. We amplified a fragment of the cytochrome b gene (1073 bp) by PCR using primers Mus 14095 and Mus 15398 (11). We used BLAST (http://blast.ncbi.nlm.nih.gov) to compare the sequence obtained (GenBank accession no. PP372564) with reference GenBank sequences and identified it as O. nigripes (98.71% BLAST identity and 100% coverage).
All seropositive rodents were active males (the sex of 1 seropositive Oligoryzomys sp. rodent was not recorded). Overall male-to-female ratio by species was 1.7:1 for A. aff. montensis and 2.3:1 for Oligoryzomys sp.
Overall seroprevalence in A. aff. montensis mice was significantly correlated (Spearman test) with richness (ρ = 0.775, p = 0.008) but not with the Shannon-Wiener diversity index (ρ = 0.261, p = 0.466), evenness index (ρ = −0.052, p = 0.886), or Simpson diversity index (ρ = 0.172, p = 0.636) (Figure 2). Overall seroprevalence of Oligoryzomys sp. rodents was not significantly correlated with any of the diversity measures (richness, ρ = −0.110, p = 0.762; Shannon-Wiener diversity, ρ = −0.372, p = 0.290; evenness, ρ = −0.164, p = 0.65; Simpson diversity, ρ = −0.277, p = 0.439) (Figure 2).
Our findings not only expand the known distribution of orthohantavirus in Misiones, Argentina, but also provide evidence of orthohantavirus infection in O. nigripes rodents in the north of this province, suggesting the presence of a pathogenic genotype in an area without known human cases. This information is relevant, particularly considering that Iguazú National Park, where 1 seropositive O. nigripes rat was captured, is visited by >1 million tourists every year.
Several pathogenic orthohantavirus have been associated with O. nigripes rodents and other Oligoryzomys spp. rodents in eastern Paraguay, southern Brazil, and northeastern Argentina (1,2,12), suggesting the seropositive animals detected in this study are probably hosts of a pathogenic genotype. However, the possibility of a spillover event from infected A. aff. montensis mice cannot be ruled out because this species is an orthohantavirus host in north Misiones (3). In fact, Oligoryzomys spp. rodents and A. aff. montensis mice were found in sympatry in all but 2 areas, suggesting the high potential for genetic reassortment and host-switching events (13), particularly in Urugua-í, where both species were found seropositive. Future studies should aim to identify the orthohantavirus genotypes in these hosts.
Although the male-to-female ratio was close to 2:1 for both species, the fact that all seropositive rodents were reproductively active males supports the role of sex in orthohantavirus transmission (1,3,14). Seroprevalence in A. aff. montensis mice was positively correlated with richness. However, that evidence is weak because of the low number of sites with seropositive rodents and was not supported by any other diversity measure.
The low overall seroprevalence detected in this study suggests HCPS risk is low in Misiones Province. However, the capacity of cricetid populations to peak unexpectedly under certain conditions (14,15), in addition to the evidence of orthohantavirus circulation in northern and central Misiones, highlight the potential risk and the need to continue surveillance.
Dr. Vadell is a CONICET research biologist at the Instituto Nacional de Medicina Tropical in Misiones, Argentina. Her interests focus on wildlife ecology and understanding pathogen-host interactions, particularly rodent and bat-borne viruses.
Acknowledgments
We are thankful to all park guards and employees in the study areas for their hospitality and collaboration and to all field assistants who helped us in fieldwork, particularly Nilso Molina, Ramón Sosa, Santiago Carrizo, and Milagros Galotta. We would also like to thank Administración de Parques Nacionales (National Parks Administration) and Ministerio de Ecología y Recursos Renovables de Misiones (Ecology and Renewable Resources Ministry of Misiones Province) for granting us support and authorization to work in the field. We also thank Laurie Bonneville for her help with English grammar. We are grateful for the dedicated comments and suggestions of the anonymous reviewers, which helped us to enrich this manuscript.
We dedicate this article to the memory of our coauthor, Laura Tauro, who died May 23, 2023.
Organizations and institutions that provided funding for this research: Agencia Nacional de Promoción Científica y Tecnología (PICT 2018-#1652, PICT-2020-#01910, PICT 2019-#02710), Universidad de Buenos Aires (UBACyT 2018-#20-20020170100171BA), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 2015-#11220150100536CO; PIBAA 2022-2023 #2872021 0101231), Ministerio de Salud de la Nación Argentina (FOCANLIS 2019 - NRU 1907), Instituto Nacional de Medicina Tropical, and Fundación Temaikèn.
References
- Vadell MV. Hantaviruses—a concise review of a neglected virus. In: Ahmad SI, editor. Human viruses: diseases, treatments and vaccines. Cham (Switzerland): Springer; 2021. p. 387–407.
- Padula P, Martinez VP, Bellomo C, Maidana S, San Juan J, Tagliaferri P, et al. Pathogenic hantaviruses, northeastern Argentina and eastern Paraguay. Emerg Infect Dis. 2007;13:1211–4. DOIPubMedGoogle Scholar
- Burgos EF, Vadell MV, Bellomo CM, Martinez VP, Salomon OD, Gómez Villafañe IE. First evidence of Akodon-borne orthohantavirus in northeastern Argentina. EcoHealth. 2021;18:429–39. DOIPubMedGoogle Scholar
- Chesini F, Brunstein L, Perrone M, Orman M, Gazia M, Gómez A, et al. Climate and health in Argentina: diagnosis of the situation 2018 [in Spanish] [cited 2023 Nov 22]. https://repositorio.smn.gob.ar/handle/20.500.12160/1223
- National Health Ministry of Argentina. Integrated Surveillance Report No. 106. Epi-week no 4 January 2012 [in Spanish] [cited 2023 Sep 12]. https://bancos.salud.gob.ar/sites/default/files/2020-01/boletinintegradodevigilanciaversion_n106-se04.pdf
- Pinto Junior VL, Hamidad AM, Albuquerque Filho DO, dos Santos VM. Twenty years of hantavirus pulmonary syndrome in Brazil: a review of epidemiological and clinical aspects. J Infect Dev Ctries. 2014;8:137–42. DOIPubMedGoogle Scholar
- World Bank. Demombynes G, Verner D. The invisible poor: a portrait of rural poverty in Argentina. Washington: World Bank Publications; 2010.
- National Health Ministry of Argentina. Basic indicators. 26th edition [in Spanish]. Buenos Aires: Ministerio de Salud y Organización Panamericana de la Salud; 2022.
- Gibbons CL, Mangen M-JJ, Plass D, Havelaar AH, Brooke RJ, Kramarz P, et al.; Burden of Communicable diseases in Europe (BCoDE) consortium. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health. 2014;14:147. DOIPubMedGoogle Scholar
- Padula PJ, Rossi CM, Valle MOD, Martínez PV, Colavecchia SB, Edelstein A, et al. Development and evaluation of a solid-phase enzyme immunoassay based on Andes hantavirus recombinant nucleoprotein. J Med Microbiol. 2000;49:149–55. DOIPubMedGoogle Scholar
- Anderson S, Yates TL. A new genus and species of phyllotine rodent from Bolivia. J Mammal. 2000;81:18–36. DOIGoogle Scholar
- Eastwood G, Camp JV, Chu YK, Sawyer AM, Owen RD, Cao X, et al. Habitat, species richness and hantaviruses of sigmodontine rodents within the Interior Atlantic Forest, Paraguay. PLoS One. 2018;13:
e0201307 . DOIPubMedGoogle Scholar - Klempa B. Reassortment events in the evolution of hantaviruses. Virus Genes. 2018;54:638–46. DOIPubMedGoogle Scholar
- de Oliveira RC, Guterres A, Fernandes J, D’Andrea PS, Bonvicino CR, de Lemos ER. Hantavirus reservoirs: current status with an emphasis on data from Brazil. Viruses. 2014;6:1929–73. DOIPubMedGoogle Scholar
- Douglass RJ, Vadell MV. How much effort is required to accurately describe the complex ecology of a rodent-borne viral disease? Ecosphere. 2016;7:
e01368 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 18, 2024
Table of Contents – Volume 30, Number 7—July 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
María Victoria Vadell, Instituto Nacional de Medicina Tropical, Puerto Iguazú, Misiones, 3370, Argentina
Top