Volume 30, Number 9—September 2024
Research Letter
Confirmed Case of Autochthonous Human Babesiosis, Hungary
Abstract
We report a case of autochthonous human babesiosis in Hungary, confirmed by PCR and partial sequencing of the Babesia spp. 18S rRNA gene. Babesiosis should be considered during the differential diagnosis of febrile illnesses, and peripheral blood smears to detect Babesia spp. should be part of the routine clinical workup.
Since the first description of human babesiosis caused by Babesia divergens protozoa in 1956 in the former Yugoslavia, 2 other zoonotic species, B. venatorum and B. microti, have been isolated in Europe. Unlike in North America, where most identified human cases have been caused by B. microti, the predominent pathogen causing babesiosis in Europe is B. divergens (1). The rising number of identified human infections in Europe has drawn attention to this emerging tickborne zoonotic disease. In Europe, B. microti has been identified in 25 of 71 confirmed human babesiosis cases, of which 11 were autochthonous (1). Rodents and insectivores are reservoir hosts for Babesia spp. parasites, which are transmitted by widespread Ixodes ricinus ticks, well-known vectors of other zoonotic pathogens (2). We report a confirmed case of human babesiosis caused by B. microti infection in Hungary.
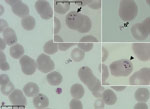
A 64-year-old man who lived in the countryside and worked as a farmer sought care at an emergency department on July 7, 2021, because of fatigue, nausea, vomiting, and a 10-kg weight loss during the past 2 months. His body temperature reached 38.9°C. He was unaware of having any chronic illnesses or tick infestations and did not have a blood transfusion or indicate a travel history outside of Hungary. Routine laboratory tests confirmed slightly elevated bilirubin (2.13 mg/dL), lactate dehydrogenase (760 U/L), alkaline phosphatase (170 U/L), gamma-glutamyl transferase (207 U/L), blood urea nitrogen (36.96 mg/dL), and creatinine (1.27 mg/dL) levels. He also had hyponatremia (120 mEq/L), prominent elevation of ultrasensitive C-reactive protein (218.8 mg/L), and new-onset diabetes, as well as slight anemia (hematocrit 37.9%) and an elevated procalcitonin level (1.94 ng/mL). A complete blood count examined by using an automated hematology analyzer (Sysmex, https://www.sysmex.com) showed elevated monocyte levels (25%) and thrombocytopenia (78 × 109 platelets/L). We examined peripheral blood smears by using automated and light microscopy, which confirmed intraerythrocytic ring forms with central vacuoles, some intraerythrocytic tetrades, and extraerythrocytic forms, suggesting babesiosis rather than malaria (Figure 1). Parasites infected 4.5% of erythrocytes. Because of microscopic findings and laboratory results, hospital staff tested blood haptoglobin level, which was 0.0 mg/dL, confirming suspected hemolysis. Other symptoms appeared during hospitalization, including left subcostal pain, decreased exercise tolerance, constipation, shivering, and new-onset torpidity; blurred vison occurred a few days after admission. Although malaria is not endemic in Hungary, we performed serologic tests for Plasmodium spp., which had negative results.
We performed PCR with primers BJ1 and BN2 to amplify a 459-bp fragment of the 18S rRNA gene of Babesia sp (3). at the University of Veterinary Medicine, Budapest; the fragment was sequenced at the University of Szeged, Szeged, Hungary. We deposited the sequence in GenBank (accession no. OP143843.1). Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov) analysis of the sequence showed 100% homology to B. microti detected in I. ricinus ticks and human blood (4). Although the sequence likely represents B. microti, further sequencing was not possible, and another closely related Babesia species cannot be excluded. We constructed a phylogenetic tree to compare the sequence with other Babesia spp. sequences found in GenBank (Figure 2).
Because of the advanced age of the patient and the clinical picture, we administered atovaquone/proguanil and azithromycin for 2 weeks (atovaquone is available in Hungary as an antimalaria drug). The patient became afebrile, and his condition improved. During follow-up examinations, fatigue and blurred vision gradually disappeared, and laboratory results improved. On the eighth day after treatment ended, we were unable to see any parasites through microscopic examination of blood. By the ninth week after treatment, lactate dehydrogenase, haptoglobin, and hemoglobin levels had normalized, and the patient fully recovered.
Although most human babesiosis cases have been imported in Europe, an increasing number of autochthonous Babesia spp. infections have been reported, possibly from a greater chance of tick contact because of human behavior changes (e.g., seeking outdoor activities) and a surge in vector population because of climate change. Furthermore, the number of immunocompromised hosts who have a more severe disease course and seek medical care is increasing as well.
In conclusion, although the 2 zoonotic species B. divergens and B. microti and their I. ricinus tick vector can be found in Hungary (8), imported or autochthonous human babesiosis cases had not been reported in this country. Babesiosis is not an endemic disease in Hungary; thus, clinicians rarely suspect this disease, despite the typical symptoms. Seroepidemiologic findings confirm the possibility of Babesia spp. transmission to humans in Europe. The increasing number of reported cases indicates that babesiosis should be considered in the differential diagnosis of patients manifesting fever in Europe. Furthermore, peripheral blood smears to detect this parasite should be a routine part of the workup for febrile illnesses, especially when disease-typical laboratory findings are present.
Dr. Sipos is a medical doctor specializing in infectious diseases. His academic interests focus on infections of immunocompromised hosts and differential diagnosis of fever.
References
- Hildebrandt A, Zintl A, Montero E, Hunfeld KP, Gray J. Human babesiosis in Europe. Pathogens. 2021;10:1165. DOIPubMedGoogle Scholar
- Yabsley MJ, Shock BC. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int J Parasitol Parasites Wildl. 2012;2:18–31. DOIPubMedGoogle Scholar
- Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13:65–70.PubMedGoogle Scholar
- Moniuszko-Malinowska A, Swiecicka I, Dunaj J, Zajkowska J, Czupryna P, Zambrowski G, et al. Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect Dis (Lond). 2016;48:537–43. DOIPubMedGoogle Scholar
- Xu S, Li L, Luo X, Chen M, Tang W, Zhan L, et al. Ggtree: A serialized data object for visualization of a phylogenetic tree and annotation data. iMeta. 2022;1:
e56 . DOIPubMedGoogle Scholar - Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. DOIPubMedGoogle Scholar
- Schliep K, Potts AJ, Morrison DA, Grimm GW. Intertwining phylogenetic trees and networks. Methods Ecol Evol. 2017;8:1212–20. DOIGoogle Scholar
- Sréter T, Kálmán D, Sréterné Lancz Z, Széll Z, Egyed L. [Babesia microti and Anaplasma phagocytophilum: two emerging zoonotic pathogens in Europe and Hungary]. Orv Hetil. 2005;146:595–600.PubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: August 09, 2024
Table of Contents – Volume 30, Number 9—September 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Dávid Sipos, University of Pécs, Rákóczi út 2, 7623 Pécs, Hungary
Top