Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 31, Number 5—May 2025
CME ACTIVITY - Research
Nationwide Observational Case–Control Study of Risk Factors for Aerococcus Bloodstream Infections, Sweden
Figure 1
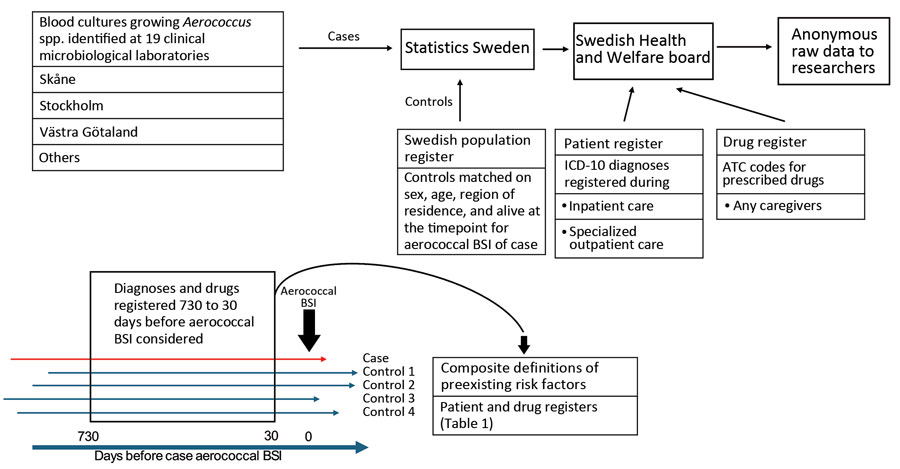
Figure 1. Overview of data collection and curation in nationwide observational case–control study of risk factors for Aerococcus BSIs, Sweden. Cases of aerococcal BSI were identified at 19 clinical microbiological laboratories across Sweden during 2012–2016. Matched control data were obtained from the Swedish Population Register. Registered diagnoses were collected from the National Patient Register, and prescribed drug data were collected from the National Drug Register. Registrations performed 30–730 days before aerococcal BSI detection were used to define medical conditions and characteristics hypothesized to contribute to aerococcal BSI. ATC, Anatomic Therapeutic Chemical; BSI, bloodstream infection; ICD-10, International Classification of Diseases, 10th Revision.