Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 31, Number 5—May 2025
Dispatch
Clade Ia Monkeypox Virus Linked to Sexual Transmission, Democratic Republic of the Congo, August 2024
Suggested citation for this article
Abstract
Several concurrent mpox outbreaks are ongoing in the Democratic Republic of the Congo. We report a case of severe clade Ia mpox in an adult woman with indeterminate HIV status who died 16 days after symptom onset. She self-identified as a sex worker and had spent time in the capital city, Kinshasa.
Mpox, a zoonotic viral disease caused by monkeypox virus (MPXV), is endemic in forested regions of central and western Africa. In recent years, disease burden has increased notably in mpox-endemic areas, alongside rapid geographic spread to nonendemic regions worldwide (1).
Historically, clade I mpox outbreaks have been predominantly driven by zoonotic transmission (2). In 2024, we reported a cluster of clade I mpox cases associated with sexual contact in Kwango Province, Democratic Republic of the Congo (DRC) (3). Although this outbreak subsided spontaneously, the emergence of clade Ib in eastern DRC has been associated with sustained human-to-human transmission (4,5). In parallel, clade Ia MPXV, associated primarily with zoonotic transmission, has been responsible for most mpox cases in DRC during the current public health emergency and has spread rapidly across the country.
Genomic investigations of mpox cases in 2024 found clades Ia and Ib in Kinshasa Province, a large urban center with international connectivity (6). This situation raises concerns about the potential public health effects on zoonotic and nonzoonotic transmission of clade Ia. In this study, we describe a fatal case of mpox caused by clade Ia, suspected to have been acquired through sexual contact, in a woman returning to Kwango from Kinshasa. Samples were collected under routine mpox surveillance, exempt from ethical approval. The Ethics Committee of Kinshasa School of Public Health (ESP-UNIKIN, nos. ESP/CE/05/2023 and ESP/CE/47/2023) and the Health Research Ethics Board at the University of Manitoba (HS25837) approved data use for this publication.
A woman (>20 years of age) with no noteworthy known medical history was transferred from a local health center to a reference hospital because of generalized cutaneous pustule-like lesions that appeared in mid-2024. She had no history of smallpox vaccination. The patient self-identified as a sex worker and had recently returned from a 6-month stay in Kinshasa, where she had multiple casual sexual partners. She reported no recent contact with persons with confirmed or suspected mpox and no recent contact with wildlife. Her first symptom was fever, followed by vulval rash and itching, which began in Kinshasa. Acetaminophen, amoxicillin, and clotrimazole vaginal pessaries were given, but no diagnostic tests were performed. Observing no improvement 4 days after symptoms started, she returned home from Kinshasa by bus. She consulted a local health center 2 days later for fever, joint pain, and generalized cutaneous and genital lesions. She was admitted and treated with ceftriaxone, vitamin C, and cetirizine before transfer 3 days later by motorcycle to the reference hospital.
At admission, clinical examination revealed fever, joint tenderness, and generalized and genital pustular lesions. Suspecting mpox, the physician placed the patient in isolation. HIV rapid diagnostic test results in 2 different health facilities were discordant. The first test at the health center was negative using the Determine HIV-1/2 kit (Abbott, https://www.abbott.com). The second test, performed 3 days later at the hospital using Uni-Gold kit (Trinity Biotech, https://www.trintybiotech.com), returned a positive result. A PCR test for HIV was not performed (Table). She was treated with artemisinin-based combination therapy for malaria and antibiotic drugs (ceftriaxone followed by lincocin) and topical antiseptic solutions to prevent secondary bacterial infections arising from skin lesions. She also received oxygen therapy for signs of respiratory distress, acetaminophen for fever, and intravenous fluids for dehydration. Despite those interventions, the patient died 16 days after symptom onset.
A case investigation team from INRB (Institut National de Recherche Biomédicale) was mobilized during a monitoring and surveillance visit on the day the patient died. The patient was in respiratory distress and had lesions at varying stages of development, some with crusting, leading to a diagnosis of mpox complicated by acute respiratory distress syndrome. Counting lesions manually was not possible because of the generalized distribution and high number. Crust and vesicle swab samples were collected and sent to INRB for diagnosis. Mpox was confirmed by PCR using pan-orthopoxvirus and MPXV-generic primers (7,8). PCR results indicated amplification cycle threshold values of 16.08 in crust samples and 15.89 in vesicle samples for the pan-orthopoxvirus primers and cycle threshold values of 14.45 in crust samples and 15.80 in vesicle samples for the MPXV-generic primers (using the same samples).
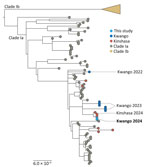
After PCR confirmation, we performed whole-viral genome sequencing using the clade IIb tiling sequencing protocol (https://www.protocols.io/view/monkeypox-virus-multiplexed-pcr-amplicon-sequencin-5qpvob1nbl4o/v2) and prepared the library on the basis of the Illumina DNA Prep protocol. We loaded the final enriched libraries onto an Illumina iSeq100 (https:/www.illumina.com). We generated MPXV consensus genomes by processing FASTQ files using CZid pipeline (https://czid.org); we used clade I MPXV genome (GenBank accession no. NC_003310.1) as reference. We used the Nextclade online tool (https://clades.nextstrain.org) to assign the clade of MPXV genomes. In addition, we used SQUIRREL to align sequences (https://github.com/aineniamh/squirrel) and inferred a maximum-likelihood phylogeny using IQ-TREE version 2.1.4 (https://github.com/Cibiv/IQ-TREE) with the Kimura 3-substitition plus empirical base frequencies plus invariant sites model as the best fit. Branch support was estimated by the ultrafast bootstrap approximation with 10,000 replicates (9). The phylogenetic tree showed that both sequences from the patient were closely related and cluster with clade Ia MPXV sequences from the ongoing outbreak in Kinshasa (Figure). This finding is consistent with severe mpox caused by clade Ia MPXV acquired during the patient’s stay in Kinshasa.
As part of routine contact tracing, 37 contacts were identified: 10 family members, 4 healthcare workers, 20 friends, and 3 sexual partners; 19 were high-risk contacts. No clinical symptoms of mpox were identified among contacts after 21 days of follow-up.
This case report describes fatal mpox caused by clade Ia MPXV in a woman with indeterminate HIV test results and early onset of genital symptoms. This finding could represent HIV seroconversion syndrome in the context of mpox infection, given the history of sexual contacts, clinical symptoms, location of lesions, and discordant HIV test results. In this resource-limited setting, details from clinical examination and laboratory investigations were insufficient to exclude other comorbidities. A false-positive HIV test result is also possible, given the low prevalence of HIV in the general population (15–49 years of age) in the DRC (0.5%–0.7%) (10). HIV prevalence of 7.5% was estimated among the key population of sex workers in 2023 (10). Given that the sex worker population might be ≈1% in DRC (11), this group might be at risk for poor outcomes from mpox, especially if HIV testing rates are low (12). PCR testing confirmed the mpox diagnosis, and whole-viral genome sequencing identified clade Ia MPXV.
This report highlights the potential severity of mpox in DRC and the need to mobilize the community to mitigate spread. The patient was a sex worker and had lived in Kinshasa, from where we reported co-circulation of clades Ia and Ib MPXV during July–August 2024 (6). This fact underscores increasing concerns about the expansion of MPXV in DRC, including to a large urban center with extensive regional and international connections, and the changing epidemiology for clade I MPXV. Although MPXV transmission through sexual contact has been predominantly associated with clade Ib (4), this case of clade Ia MPXV infection might have been linked to sexual contact, as evidenced by presence of genital lesions. The findings are consistent with our previous report of a cluster of mpox cases associated with sexual contacts in Kwango Province, which also involved clade Ia (3).
In summary, this report highlights the importance of prompt diagnosis and public health intervention, specifically among high-risk groups, to prevent the spread of mpox, as well as early diagnosis and management of HIV infection. In regions where mpox is emerging, healthcare providers must maintain a high index of suspicion, particularly in patients with vesiculopustular rash and systemic symptoms.
Dr. Makangara-Cigolo is a doctoral student at Graduate School of Cellular and Biomedical Sciences, University of Bern, Switzerland. His research interests include emerging and reemerging viral diseases with a focus on genome sequencing, bioinformatics, and public health interventions.
Acknowledgments
We thank the Provincial Health Division of Kwango and the local team supporting the International Mpox Research Consortium activities, including Tshotsho Tshonaka Dixon, Seraphin Tseke, Lucien Lufutu, Judith Mayamba, Guillaume Nduka, and Mundos Mwayi. We also acknowledge all the staff of INRB, particularly those from the Virology Department and the Epidemiology and Global Health Department. We also gratefully acknowledge the support provided by the US Centers for Disease Control and Prevention Poxvirus and Rabies Branch (Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases) during the ongoing mpox public health emergency of international concern as well as their historic support for mpox surveillance in the region.
The project or effort depicted is sponsored by the International Mpox Research Consortium through funding from the Canadian Institutes of Health Research and International Development Research Centre (grant no. 202209MRR-489062-MPX-CDAA-168421). Additional support was provided in part by the Department of Defense Threat Reduction Agency (HDTRA1-21-1-0040). This work is also supported by the US Department of Agriculture (Agriculture Research Service (Non-Assistance Cooperative Agreement no. 20230048, grant no. 58-3022-2-020). The content of the information does not necessarily reflect the position or the policy of the United States or Canada governments, and no official endorsement should be inferred. The Africa Pathogen Genomics Initiative helped acquiring and maintaining the sequencer; Agence Française de Développement through the AFROSCREEN project (grant agreement CZZ3209, coordinated by ANRS Maladies infectieuses émergentes in partnership with Institut de Recherche pour le Développement and Pasteur Institute) for laboratory support and PANAFPOX project funded by ANRS Maladies infectieuses émergentes; Belgian Directorate-general for Development Cooperation and Humanitarian Aid, the Department of Economy, Science, and Innovation of the Flemish government, the Research Foundation–Flanders (FWO, grant no. G096222 N to L.L.), and EDCTP grant no. 101195465 (MBOTE-SK); and US National Institute of Allergy and Infectious Diseases/National Institutes of Health grant no. U01AI151799 through Center for Research in Emerging Infectious Disease-East and Central Africa. We acknowledge the support of the Wellcome Trust (Collaborators Award 206298/Z/17/Z, ARTIC network) and the CIZD group. T.W.B. acknowledges funding from the European Union (FORTIFIEDx project under the Horizon Europe research and innovation programme and grant agreement no. 101092049). Views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union or the granting authority European Union’s Horizon Europe research and innovation programme. Neither the European Union nor the granting authority can be held responsible for them.
J.K., T.W.B., and P.M.K. conceived, designed, and supervised the study. J.-C.M.-C., K.M.K., L.L., Y.A., S.K., D.J., E.K.L., A.A.A., E.M., P.A.B., E.L.L., R.L.N., G.K.L., G.L., J.J.M.T., S.A.M., T.W.B., and P.M.K. acquired the data. J.-C.M.-C., K.M.K., L.L., Y.A., S.K., D.J., E.K.L., S.K., A.A.A., E.M., P.A.B., E.L.L., E.H.V., S.S.N., E.P.S., P.P.T., R.O.M., R.L.N., G.K.L., G.L., Á.O., D.M.B., N.A.H., N.L., A.W.R., J.J.M.T., A.R., K.V., S.A.M., J.K., T.W.B., and P.M.K. analyzed and interpreted data. J.C.M.C. and K.M.K. wrote the first draft, and J.K., T.W.B., and P.M.K. reviewed that draft. J.C.M.C., K.M.K., L.L., S.K., D.J., E.K.L., S.K., A.A.A., E.M., Y.A., P.A.B., E.L.L., E.H.V., S.S.N., E.P.S., P.P.T., R.O.M., R.L.N., G.K.L., G.L., C.K., R.S.L., D.M., Á.O., S.M., M.H., D.M.B., N.A.H., N.L., L.S., L.L., A.A., M.P., E.D., S.T., A.W.R., J.J.M.T., A.R., K.V., S.A.M., J.K., T.W.B., and P.M.K. revised the submitted article
References
- Alakunle E, Kolawole D, Diaz-Cánova D, Alele F, Adegboye O, Moens U, et al. A comprehensive review of monkeypox virus and mpox characteristics. Front Cell Infect Microbiol. 2024;14:
1360586 . DOIPubMedGoogle Scholar - Van Dijck C, Hoff NA, Mbala-Kingebeni P, Low N, Cevik M, Rimoin AW, et al. Emergence of mpox in the post-smallpox era-a narrative review on mpox epidemiology. Clin Microbiol Infect. 2023;29:1487–92. DOIPubMedGoogle Scholar
- Kibungu EM, Vakaniaki EH, Kinganda-Lusamaki E, Kalonji-Mukendi T, Pukuta E, Hoff NA, et al.; International Mpox Research Consortium. Clade I–associated mpox cases associated with sexual contact, the Democratic Republic of the Congo. Emerg Infect Dis. 2024;30:172–6. DOIPubMedGoogle Scholar
- Vakaniaki EH, Kacita C, Kinganda-Lusamaki E, O’Toole Á, Wawina-Bokalanga T, Mukadi-Bamuleka D, et al. Sustained human outbreak of a new MPXV clade I lineage in eastern Democratic Republic of the Congo. Nat Med. 2024;30:2791–5. DOIPubMedGoogle Scholar
- Hoffmann C. Mpox-is there a more dangerous new clade? Lancet Infect Dis. 2024;24:
e667 . DOIPubMedGoogle Scholar - Wawina-Bokalanga T, Akil-Bandali P, Kinganda-Lusamaki E, Lokilo E, Jansen D, Amuri-Aziza A, et al. Co-circulation of monkeypox virus subclades Ia and Ib in Kinshasa Province, Democratic Republic of the Congo, July to August 2024. Euro Surveill. 2024;29:
2400592 . DOIPubMedGoogle Scholar - Schroeder K, Nitsche A. Multicolour, multiplex real-time PCR assay for the detection of human-pathogenic poxviruses. Mol Cell Probes. 2010;24:110–3. DOIPubMedGoogle Scholar
- Li Y, Zhao H, Wilkins K, Hughes C, Damon IK. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169:223–7. DOIPubMedGoogle Scholar
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 2018;35:518–22. DOIPubMedGoogle Scholar
- World Health Organization. Democratic Republic of the Congo HIV country profile 2023 [cited 2024 Oct 10]. https://cfs.hivci.org
- UNAIDS. UNAIDS data 2022 [cited 2024 Oct 10]. https://www.unaids.org/en/resources/documents/2023/2022_unaids_data
- Migaud P, Naidenow J, Ghaeni L, Hosmann K, Kaghoma J, Kowomba J, et al. Indicator-condition-guided HIV testing in rural DRC. Infection. 2024;52:1171–4. DOIPubMedGoogle Scholar
Figure
Table
Suggested citation for this article: Makangara-Cigolo J-C, Kenye K-M, Lunyanga L, Jansen D, Kinganda-Lusamaki E, Kavira S, et al. Clade Ia monkeypox virus linked to sexual transmission, Democratic Republic of the Congo, August 2024. Emerg Infect Dis. 2025 May [date cited]. https://doi.org/10.3201/eid3105.241690
Original Publication Date: April 08, 2025
1These authors contributed equally to this article.
2These authors jointly supervised this study.
Table of Contents – Volume 31, Number 5—May 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Placide Mbala-Kingebeni, Institut National de Recherche Biomédicale, Department of Epidemiology and Global Health, Avenue de la Démocratie, Kinshasa/Gombe, B.P. 1197 Kinshasa 1, Democratic Republic of the Congo
Top