Volume 6, Number 1—February 2000
Synopsis
Dengue Surveillance in Florida, 1997-98
Abstract
Recent dengue outbreaks in the Caribbean and Central and South America and the presence of competent mosquito vectors increase the likelihood of future autochthonous transmission in Florida. During April 1997 to March 1998, a laboratory-based active surveillance program detected 18 cases of dengue involving all four dengue serotypes. All patients reported recent travel to countries with indigenous dengue transmission. These results demonstrate that dengue infections are imported into Florida at a much higher rate than reflected by previous passive surveillance; therefore, the risk for local dengue transmission may be increasing.
Dengue is a mosquito-borne viral disease caused by one of four antigenically distinct dengue flaviviruses: DEN-1, DEN-2, DEN-3, and DEN-4. Primary infection with any serotype may lead to acute illness defined as fever with two or more of the following symptoms: headache, retroorbital pain, myalgia, arthralgia, rash, and hemorrhagic manifestations (1,2). Fever and other symptoms may subside after 3 or 4 days, and the patient may recover completely, or the fever may return with a rash within 1 to 3 days (3). Secondary exposure to the same serotype generally does not produce illness because of pre-existing antibodies. However, secondary exposure to a different serotype may lead to another dengue fever episode, and the patient may be at risk for more serious forms of infection, dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) (3). Dengue virus infection may also cause a nonspecific febrile illness that can be easily confused with measles or influenza. Therefore, laboratory testing is essential to clinical diagnosis and public health reporting.
Dengue-viremic persons are usually infectious to the mosquito vector 1 day before the onset of the febrile period and remain so for 6-7 days. When a mosquito ingests virus in a blood meal, the virus replicates during an extrinsic incubation period of 8 to 12 days, after which the mosquito remains infective for life (4,5). The life span of Aedes aegypti, the primary vector of dengue in the Americas, is usually 21 days, although life span and incubation periods depend on temperature and rainfall (6). Both A. aegypti and A. albopictus, a recently introduced vector species (7), have been found throughout Florida, and A. aegypti breeds year-round in south Florida (8).
The last dengue epidemics in Florida, in the Tampa and Miami areas in 1934-35 (9,10), affected an estimated 15,000 of the 135,000 population of Miami. The last recorded epidemic in the southeastern states was in Louisiana in 1945 (11). Most cases of dengue reported in the United States since the 1940s have been imported; however, indigenous transmission of dengue occurred in Texas in 1986 and 1995 (8,12-14). In response to an outbreak of dengue in Mexico in 1995, the Texas Department of Health initiated an active surveillance program that detected 29 confirmed cases, including seven in persons with no recent history of travel outside Texas (14,15). Thirteen imported dengue cases (0 to 4 cases per year) were reported in Florida from 1985 to 1995 (16).
The recent introduction of DEN-3 in Mexico and Central America is of public health importance because most of the population in the tropical Americas is susceptible to infection with this serotype (17,18). The presence of the vector, the rapid spread of the virus, and increased air travel and immigration contribute to the possibility of future dengue transmission in the continental United States (19-21). A serosurvey conducted after the first confirmed dengue outbreak in Peru in 1990 clearly demonstrated earlier undetected dengue transmission (22). Silent transmission of dengue was also demonstrated in 1992 in an area of Taiwan believed free of the disease (23). In both cases, an early warning system based on immunoglobulin (Ig)M antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA) laboratory tests was recommended for disease monitoring. Active surveillance, an essential component of an early warning system for detection of dengue, provides information vital to defining epidemiologic aspects of cases and enabling educational and mosquito control efforts (24-27).
Recent outbreaks of dengue in nearby Caribbean and Central and South American countries may increase the likelihood of future autochthonous transmission in Florida (15). Mosquito vectors are widely distributed in the state, and travelers returning from dengue-endemic areas place at risk the resident population, which has minimal (if any) immunity to dengue viruses. Because physicians' awareness of dengue is low and specialized laboratory diagnostic methods are not available locally, low-level dengue transmission may go undetected. Imported dengue may thus be underreported in Florida, which has relied on passive surveillance. We used an educational campaign for county epidemiologists and health-care providers and an active laboratory-based surveillance program that facilitated prompt, accurate diagnosis of dengue to assess the risk for local dengue fever transmission in Florida.
The first phase of the surveillance program was the design of a dengue information packet for all 67 county health department epidemiologists in Florida, to be distributed to hospital emergency rooms, clinics, health departments, and infectious disease physicians in the county. The letter included information on case reporting, the dengue case definition, specimen requirements and transport instructions, and a dengue case investigation form.
Under cooperative agreements with two Florida commercial clinical laboratories (national reference clinical laboratories), specimens from patients with suspected dengue were forwarded to the state laboratory for free testing. In cases where specimens were tested at commercial laboratories only, dengue antibody-positive results were forwarded to county health departments and to the state laboratory for inclusion in this study. In Florida, dengue testing is offered only by the state laboratory and some commercial clinical laboratories.
Before this study, the hemagglutination inhibition (HAI) assay was the only serologic test for dengue offered at the state laboratory. Laboratory capabilities were enhanced to include testing for IgM antibodies to dengue. Acute- and convalescent-phase serum specimens were tested for dengue antibodies by both HAI assay and MAC-ELISA, using a DEN1-4 serotype cocktail (28-30). Available specimens positive for IgM antibodies to dengue, tested at the Florida state laboratory, were forwarded to CDC's Dengue Branch laboratory for virus isolation, serotyping, and confirmation of serologic results.
Cases were classified as DHF if all the following were present: fever, hemorrhagic tendencies, thrombocytopenia (100,000/mm3 or less), and evidence of plasma leakage (hematocrit level increased by > 20%) or other objective evidence of increased capillary permeability (31). If all the above were present, plus hypotension or pulse pressure < 20 mm Hg, the case was classified as DSS.
In this study, a case was classified as presumptive dengue on the basis of serologic evidence of an HAI titer > 1:1280, an equivalent IgG titer, or a positive dengue IgM antibody test on a single serum sample. A confirmed dengue case required a fourfold rise in HAI, IgG, or IgM antibody titers between acute- and convalescent-phase serum specimens; isolation of virus; or detection of viral antigen by immunohistochemistry, immunofluorescence, or viral nucleic acid detection. Confirmed or presumptive dengue cases are referred to as laboratory-diagnosed cases.
A case was classified as undetermined if sufficient information was not available on the timing of specimen collection in relation to onset of symptoms or a convalescent-phase serum was not available to demonstrate a fourfold rise in antibody titers. A case was also considered undetermined if the acute-phase serum was negative for antibodies and a convalescent-phase serum was not available.
Epidemiologic data were obtained from dengue case investigation forms that accompanied the patients' specimens. Suspected as well as confirmed cases of dengue are reportable in Florida (32). County health departments were notified of suspected cases, and a convalescent-phase serum was requested.
We used the Epi Info Software package for data analysis (33). Comparisons were made with historical data on reported cases of dengue (9,16,34-36).
From April 1, 1997, to March 31, 1998, 83 suspected cases of dengue were studied. Commercial clinical laboratories referred specimens for analysis for 36 (43%) of these cases. The rest were referred through county health departments, hospital laboratories, infection control practitioners, or physicians. Recent dengue infection was laboratory diagnosed in 18 (22%) of these cases. Twelve (67%) of the 18 confirmed dengue specimens were referred by commercial clinical laboratories. Virus isolation or polymerase chain reaction of five cases yielded all four dengue serotypes. Dengue was ruled out as the etiologic agent in 24 (29%) cases. The remaining 41 (49%) cases were undetermined because convalescent-phase serum samples were not available (Table 1).
Most (65%) of suspected-dengue patients were male (chi-square goodness of fit test p value = 0.006). Among suspected cases the mean age was 41 years (1 day to 79 years). Forty-one (49%) initially tested positive for anti-flavivirus antibodies. Convalescent-phase serum was obtained from 25 (30%) of the cases. The average age of patients with confirmed dengue cases was 37 years (8 to 69); 14 (78%) of the 18 patients were male.
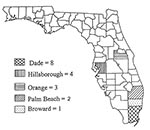
Laboratory-diagnosed cases were identified from five counties in central and extreme southeastern Florida (Figure 1). Cases were confirmed in persons residing in the following counties: Dade (8), Hillsborough (4), Orange (3), Palm Beach (2), and Broward (1). Table 2 lists Florida counties with laboratory-diagnosed dengue cases, case travel history, and dengue virus serotypes detected. All 18 laboratory-diagnosed dengue cases were in persons who had recently traveled to dengue-endemic areas and were therefore classified as imported. We included out-of-state cases in our analysis because the acute phase of their illness occurred while they were in Florida. Current county health department policy dictates that only cases in Florida residents are reported to the state epidemiologist for recording in the weekly and yearly morbidity statistics. Other case reports are forwarded to the county and state of primary residence of the patient.
Hemorrhagic manifestations were reported in 7 (39%) of the 18 confirmed cases; one met the DHF case definition; however, it was not possible to classify the remaining six cases with hemorrhage because information on hemoconcentration and plasma leakage was incomplete. Encephalopathy was present in one case. Antibody titers suggested secondary dengue infections in 10 (56%) of the 18 cases. Only 2 (11%) of the 18 cases appeared to be primary infections. Laboratory tests necessary to determine infection status (primary vs. secondary) were not available in the other six cases. A woman with acute secondary dengue infection with hemorrhagic manifestations gave birth to a healthy uninfected baby.
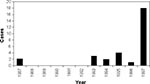
During the year of active surveillance, 18 laboratory-diagnosed cases of dengue were detected. On the basis of the previous 10-year mean of 1.3 cases per year (Figure 2), the probability of detecting 18 cases was virtually 0% (Poisson distribution rare event vs. standard test). These cases were identified in Florida counties with high rates of international travel and large immigrant populations, as well as year-round breeding of A. albopictus and A. aegypti mosquitoes. According to Florida Department of Commerce statistics, of the 6 million international visitors to Florida in 1995, 38.4% traveled from South and Central America, the Caribbean, Mexico, Asia, and other tropical areas (37) in which dengue is endemic.
All four dengue serotypes were detected in five specimens during this study. Improved specimen handling should increase the rate of virus isolation. The serotype of the infecting dengue virus was identified in only five cases for the entire United States in 1995, when 79 laboratory-diagnosed dengue cases were documented (12). In the same year, 22 imported and seven indigenous cases were detected in Texas (15). In 1996, the infecting dengue serotype was identified in 5 of the 43 laboratory-diagnosed cases of imported dengue in the United States (3 cases of DEN-1 and 2 of DEN-2) (38).
This study found multiple problems with routine clinical laboratory confirmation and follow-up of dengue infections: Tests requested by physicians and performed at clinical laboratories were not always optimal for identifying a current dengue infection. Even though the dengue IgM test is the most appropriate assay for determining current infection, it is not routinely performed at commercial laboratories and may not be readily available if requested. Test results are frequently misinterpreted, e.g., a single positive indirect fluorescent antibody test performed at a commercial laboratory may be interpreted as positive for current dengue infection when it only indicates infection with a flavivirus (e.g., dengue, St. Louis encephalitis, Japanese encephalitis) or vaccination (e.g., yellow fever) at an undetermined time in the past. In addition, cases are rarely investigated, and the convalescent-phase serum samples needed for confirmation are rarely requested. When an investigation indicates need for further testing, specimens may have already been discarded. Finally, positive test results are often not forwarded to the county and state epidemiologists in a timely manner. In cases tested only at commercial laboratories, delays of 2 to 4 months before positive cases were reported to the state Bureau of Epidemiology preclude prompt follow-up.
Three of the confirmed dengue cases in this study tested at commercial laboratories had not been reported to the state epidemiologist by the county health departments because the patients were primary residents of other states, although they became ill while in Florida.
This study indicates that surveillance efforts should be concentrated in densely populated counties with large numbers of international travelers (Dade, Palm Beach, Orange, and Hillsborough), especially during dengue season in the Caribbean (July to November). As a part of the epidemiologic investigation of imported dengue cases, an attempt should be made to identify secondary cases.
Dr. Gill is on the staff of the Florida Department of Health, Pinellas County Health Department in the Disease Control Division. She is also a Courtesy Assistant Professor in the Department of Environmental and Occupational Health, College of Public Health, University of South Florida. Her research interests include laboratory-based surveillance programs for hepatitis, dengue, and other arboviral diseases.
Acknowledgment
The authors thank the Association for Public Health Laboratories and CDC for supporting the laboratory training for this program through the Emerging Infectious Diseases Advanced Laboratory Training Fellowship and the staff of the Florida Department of Health, Bureaus of Laboratories and Epidemiology, and CDC's Dengue Branch for their collaboration in this program.
References
- Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Morb Mortal Wkly Rep. 1997;46(RR-10):45–6.
- Pan American Health Organization. Dengue and dengue hemorrhagic fever in the Americas: guidelines for prevention and control. Washington: The Organization (Scientific Pub. No. 548); 1994.
- Hayes EB, Gubler DJ. Dengue and dengue hemorrhagic fever. Pediatr Infect Dis J. 1992;11:311–7. DOIPubMedGoogle Scholar
- Benenson AS, ed. Dengue and dengue hemorrhagic fever. In: Control of communicable diseases manual. 16th ed. Washington: American Public Health Association; 1995.
- Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17:321–35.PubMedGoogle Scholar
- Focks DA, Daniels E, Hailem DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995;53:489–506.PubMedGoogle Scholar
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB. Aedes albopictus in North America: probable introduction in used tires from Northern Asia. Science. 1987;236:1114–6. DOIPubMedGoogle Scholar
- Clark GG. Dengue and dengue hemorrhagic fever. Journal of the Florida Mosquito Control Association. 1992;63:48–53.
- Griffitts THD. Dengue in Florida 1934, and its significance. J Fla Med Assoc. 1935;21:395–7.
- MacDonnell GN. The dengue epidemic in Miami. J Fla Med Assoc. 1935;21:392–8.
- Ehrenkranz NJ, Ventura AK, Cuadrado RR, Pond WL, Porter JE. Pandemic dengue in Caribbean countries and the southern United States—past, present and potential problems. N Engl J Med. 1971;285:1460–9.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Imported dengue–United States, 1995. MMWR Morb Mortal Wkly Rep. 1996;45:988–91.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Dengue activity in the Americas, 1994. MMWR Morb Mortal Wkly Rep. 1995;:44.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Dengue fever at the U.S.-Mexico Border, 1995-1996. MMWR Morb Mortal Wkly Rep. 1996;45:841–4.PubMedGoogle Scholar
- Rawlings JA, Hendricks KA, Burgess CR, Campman RM, Clark GG, Tabony LJ, Dengue surveillance in Texas, 1995. Am J Trop Med Hyg. 1998;59:95–9.PubMedGoogle Scholar
- Florida Department of Health. Florida Morbidity Statistics, 1995. Tallahassee (FL): Florida Department of Health, Bureau of Epidemiology; 1995.
- Briseño-Garcia B, Gomez-Dantes H, Argott-Ramirez E, Montesano R, Vazquez-Martinez A-L, Potential risk for dengue hemorrhagic fever: the isolation of serotype Dengue-3 in Mexico. Emerg Infect Dis. 1996;2:133–5. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Dengue-3 in Central America. Dengue Surveillance Summary 1995;70.
- Pan American Health Organization. Re-emergence of dengue in the Americas. Epidemiol Bull. 1997;18:1–10.
- Gubler DJ, Clark GG. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg Infect Dis. 1995;1:55–7. DOIPubMedGoogle Scholar
- Pan American Health Organization. Dengue and dengue hemorrhagic fever, 1996. Epidemiol Bull. 1996;17:12–4.
- Hayes CG, Phillips IA, Callahan JD, Griebenow WF, Hyams KC, Wu S-J, The epidemiology of dengue virus infection among urban, jungle, and rural populations in the Amazon region of Peru. Am J Trop Med Hyg. 1996;55:459–63.PubMedGoogle Scholar
- Chen W-J, Chen S-L, Chien L-J, Chen C-C, King C-C, Harn M-R, Silent transmission of the dengue virus in Southern Taiwan. Am J Trop Med Hyg. 1996;55:12–6.PubMedGoogle Scholar
- Gubler DJ. Aedes aegypti and Aedes aegypti-borne disease control in the 1990s: top down or bottom up. Am J Trop Med Hyg. 1989;40:571–8.PubMedGoogle Scholar
- Gubler DJ. Surveillance for dengue and dengue hemorrhagic fever. Bull Pan Am Health Organ. 1989;23:397–404.PubMedGoogle Scholar
- Gubler DJ. The global resurgence of arboviral diseases. Trans R Soc Trop Med Hyg. 1996;90:449–51. DOIPubMedGoogle Scholar
- Ruangturakit S, Rojanasuphot S, Srijuggravanvong A, Duangchanda S, Nuangplee S, Igarashi A. Storage and stability of dengue IgM and IgG antibodies in whole blood and serum dried on filter paper strips detected by ELISA. Southeast Asian J Trop Med Public Health. 1994;25:560–4.PubMedGoogle Scholar
- Gubler DJ, Sather GE. Laboratory diagnosis of dengue and dengue hemorrhagic fever. Proceedings of the International Symposium on Yellow Fever and Dengue. Rio de Janeiro, Brazil; 1988 May 15-19. Bio-Manguinhos; 1988. p. 291-322.
- Kuno G, Gomez I, Gubler DJ. Detecting artificial anti-dengue IgM immune complexes using an enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1987;36:153–9.PubMedGoogle Scholar
- Kuno G, Gomez I, Gubler D. An ELISA procedure for the diagnosis of dengue infections. J Virol Methods. 1991;33:101–13. DOIPubMedGoogle Scholar
- World Health Organization. Dengue hemorrhagic fever: diagnosis, treatment and control. Geneva: The Organization; 1986.
- Florida Department of Health and Rehabilitative Services. Florida Statutes, 1996 (Supplement 1996). Chapter 381: Public Health; General Provisions. Rule 10D-3.062.
- Centers for Disease Control. Epi Info Software Package, 1991. Version 5.0.
- Florida Department of Health and Rehabilitative Services. Florida Morbidity Statistics, 1981. Tallahassee (FL): Health Program Office; 1981.
- Florida Department of Health and Rehabilitative Services. Florida Morbidity Statistics, 1985. Tallahassee (FL): Health Program Office; 1985.
- Rigau-Perez JG, Gubler DJ, Vorndam AV, Clark GG. Dengue surveillance—United States, 1986-1992. MMWR Morb Mortal Wkly Rep. 1994;43(SS-2):7–19.
- Florida Department of Commerce. 1995 Florida visitor study. Tallahassee (FL): Bureau of Economic Analysis; 1995.
- Centers for Disease Control and Prevention. Imported dengue—United States, 1996. MMWR Morb Mortal Wkly Rep. 1998;47:544–7.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 6, Number 1—February 2000
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Julia Gill, Florida Department of Health, Pinellas County Health Department, Disease Control Division, 500 7th Avenue South, St. Petersburg, FL, 33701, USA; fax: 727-893-1681
Top