Volume 16, Number 10—October 2010
Dispatch
Artesunate Misuse and Plasmodium falciparum Malaria in Traveler Returning from Africa
Abstract
Plasmodium falciparum malaria developed in an African-born traveler who returned to Canada after visiting Nigeria. While there, she took artesunate prophylactically. Isolates had an elevated 50% inhibitory concentration to artemisinin, artesunate, and artemether, compared with that of other African isolates. Inappropriate use of artemisinin derivatives can reduce P. falciparum susceptibility.
Artemisinin derivatives were recently approved by the Food and Drug Administration for the treatment of Plasmodium falciparum malaria in North America and are available through the US Centers for Disease Control and Prevention and through Health Canada (1–3). Artemisinin-based combination therapy (ACT) remains the most effective therapy for P. falciparum malaria throughout the world, with the possible exception of the Thailand–Cambodia border (4). Because of the large numbers in the Toronto area of returning travelers and recent immigrants who have returned to countries of origin and visited friends and relatives, the Public Health Laboratory (Toronto) identifies ≈200 positive malaria smears annually; most P. falciparum isolates have come from sub-Saharan Africa. Evidence has indicated that such travelers tend not to seek medical advice before travel and are therefore at high risk of acquiring malaria (5).
A 38-year-old Nigerian-born woman, who lived in the Toronto area (and has a good ability to recount her experiences), returned to Lagos, Nigeria, for a visit in January 2009. She did not seek pretravel advice. On arrival in Lagos, the woman purchased artesunate locally and began taking two 50-mg tablets weekly for the 4 weeks of her visit. Immediately on her return to Toronto, the patient experienced myalgia, nausea with vomiting, and chills, ≈7 days after she had taken her last dose of oral artesunate. She sought treatment at the emergency department of a community hospital. Physical examination showed that her temperature was 39.1°C and that she was dehydrated. Laboratory tests showed the following: leukocyte count 3,700 cells/μL, thromocyte count 72 × 103 cells/μL, hemoglobin level 12.7 g/dL. Her chest radiograph showed that her lungs were clear. An examination of peripheral blood by thick and thin films showed a 0.7% parasitemia with P. falciparum. Her condition was treated with 1,250 mg of oral mefloquine as a single dose. She was treated as an outpatient, and she reported that symptoms promptly resolved over the next 48 hours without side effects.
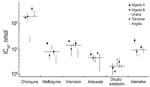
A blood specimen was placed into culture in the Public Health Laboratory (Toronto), and the P. falciparum isolate was tested for drug susceptibility (6). The 50% inhibitory concentration (IC50) was the following for certain antimicrobial agents (tested in triplicate): chloroquine 170.5 ± 7.8 nmol/L, mefloquine 16.6 ± 0.7 nmol/L, artemisinin 20.1 ± 0.6 nmol/L, artesunate 6.2 ± 1.4 nmol/L, dihydroartemisinin 1.8 ± 0.9 nmol/L, and artemether 21.4 ± 5.3 nmol/L. For this P. falciparum isolate, IC50 was significantly higher for artemisinin, artesunate, and artemether than for other representative P. falciparum isolates imported from Africa (Figure). Because of the short half-life of artesunate, the weekly doses of the oral drug may have led to development of a resistant strain when the patient was in Nigeria. Artesunate-containing drugs therefore should not be used for prophylaxis or single drug therapy. The purchased artesunate may also have been counterfeit and may have contained lower levels of active drug. Although these data suggest that this isolate has reduced susceptibility to artemisinin derivatives, the correlation between in vitro susceptibility and treatment outcomes does not appear to be consistent (4).
Previous studies have reported that resistance to artemisinin is mediated by an increase in gene copy number, mutations within the efflux pump of the P. falciparum multidrug resistance 1 (pfmdr1) gene, or mutations in the calcium transporter pfATPase6 (7,8). When we examined each gene, using a combination of real-time PCR and DNA sequencing, we found that pfmdr1 copy number was elevated in this isolate relative to that of the susceptible control strain 3D7. We also observed nonsynonymous mutations in both pfmdr1 (Y184F) and pfATPase6 (A623E, S769N), whereas other implicated residues remained in the wild-type form (9) (Table). Similar molecular analysis of other representative imported African clinical isolates demonstrated variable mutations for pfmdr1 and pfATPase6 and copy number in relation to IC50 values for key drugs (Table). A trend, albeit weak, was observed in which increased pfmdr1 copy number was correlated with an elevated IC50 to mefloquine (r = 0.52) and artemisinin (r = 0.42). The presence of an asparagine (N) at position 86 of Pfmdr1, when coupled to an elevated pfmdr1 copy number, appeared to correlate well with reduced susceptibility to artemisinin (Table). Chavchich et al. recently demonstrated that increased pfmdr1 copy number occurred in a laboratory strain placed under drug selection pressure with artemisinin derivatives (11). However, Imwong et al. have indicated that genetic polymorphisms and copy number in pfmdr1 do not predict treatment outcome with ACT (10).
Findings in the published literature vary in terms of use of artemisinin derivatives for in vitro drug susceptibility testing. Jambou et al. reported treatment failures with ACT in Cambodia, French Guiana, and Senegal (8). These authors used artemether for testing and showed IC50 values of ≈30 nmol/L in their “resistant” isolates from Senegal. Noedl et al. described treatment failures with ACT in Cambodia, for which IC50 values to dihydroartemisinin were ≈10 nmol/L (12). Dondorp et al. showed IC50 values of 4–6 nmol/L to dihydroartemisinin and 6–8 nmol/L to artesunate in a region of Cambodia and Thailand where ACT treatment failures have occurred (4). Systematic molecular surveillance and standardized drug-testing methods with clinical isolates are required to establish the molecular correlates of reduced susceptibility to antimalarial drugs. In this regard, efforts are ongoing under the auspices of the Worldwide Antimalarial Research Network (13).
The patient’s infection responded to mefloquine when she was back in Canada, possibly because of the high oral dose of mefloquine. Current guidelines from the US Centers for Disease Control and Prevention recommend quinine sulfate plus doxycycline, tetracycline, or clindamycin; or atovaquone-proguanil (Malarone; GlaxoSmithKline, Mississauga, Ontario, Canada) as first- and second-line treatment for uncomplicated P. falciparum malaria. Reduced susceptibility to artesunate is more likely to occur when it is associated with inappropriate use of artemisinin derivatives than because of circulating artemisinin-resistant P. falciparum in sub-Saharan Africa.
In an effort to achieve consensus that artesunate oral monotherapies should not be marketed, the World Health Organization convened the international pharmaceutical sector in April 2006. At that time, 15 companies agreed to cease manufacturing artesunate monotherapies. However, oral artesunate montherapies may still be purchased over the counter in malaria-endemic countries, as this report shows. Thus, strains of P. falciparum malaria are currently at risk of developing reduced susceptibility to artesunate derivatives.
Dr Pillai is a medical microbiologist at the Public Health Laboratory (Toronto), clinical associate at the University Health Network, and assistant professor of medicine at the University of Toronto. His research interests focus on reversing mechanisms of antimicrobial resistance and laboratory surveillance of infectious diseases, including malaria and Streptococcus pneumoniae and Clostridium difficile infections.
Acknowledgments
We thank the Clinical Parasitology Department at the Public Health Laboratory (Toronto) for expert technical assistance.
This work was funded by the Ontario Agency for Health Protection and Promotion.
References
- Centers for Disease Control and Prevention. Artesunate now available to treat severe malaria in the United States [cited 2010 Jun 10]. http://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html
- Centers for Disease Control and Prevention. Coartem, new malaria treatment drug, now available [cited 2010 Jun 10]. http://www.cdc.gov/malaria/diagnosis_treatment/treatment.html
- Health Canada. Special Access Programme—drugs [cited 2010 Jun 10]. http://www.hc-sc.gc.ca/dhp-mps/acces/drugs-drogues/index-eng.php
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. DOIPubMedGoogle Scholar
- Bacaner N, Stauffer B, Boulware DR, Walker PF, Keystone JS. Travel medicine considerations for North American immigrants visiting friends and relatives. JAMA. 2004;291:2856–64. DOIPubMedGoogle Scholar
- Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother. 2007;51:1926–33. DOIPubMedGoogle Scholar
- Price RN, Cassar C, Brockman A, Duraisingh M, van Vugt M, White NJ, The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob Agents Chemother. 1999;43:2943–9.PubMedGoogle Scholar
- Jambou R, Legrand E, Niang M, Khim N, Lim P, Volney B, Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–3. DOIPubMedGoogle Scholar
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real- time quantitative PCR and the 2–-∆∆CT method. Methods. 2001;25:402–8. DOIPubMedGoogle Scholar
- Imwong M, Dondorp AM, Nosten F, Yi P, Mungthin M, Hanchana S, Exploring the contribution of candidate genes to artemisinin resistance in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:2886–92. DOIPubMedGoogle Scholar
- Chavchich M, Gerena L, Peters J, Chen N, Cheng Q, Kyle DE. Role of pfmdr1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:2455–64. DOIPubMedGoogle Scholar
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20. DOIPubMedGoogle Scholar
- Plowe CV, Roper C, Barnwell JW, Happi CT, Joshi HH, Mbacham W, World Antimalarial Resistance Network (WARN) III: molecular markers for drug resistant malaria. Malar J. 2007;6:121. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 16, Number 10—October 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Dylan R. Pillai, 81A Resources Rd, Rm 243, Toronto, ON M9P 3T1, Canada
Top