Volume 16, Number 2—February 2010
Dispatch
White-Nose Syndrome Fungus (Geomyces destructans) in Bat, France
Abstract
White-nose syndrome is caused by the fungus Geomyces destructans and is responsible for the deaths of >1,000,000 bats since 2006. This disease and fungus had been restricted to the northeastern United States. We detected this fungus in a bat in France and assessed the implications of this finding.
Biologists are struggling to understand a recent emerging infectious disease, white-nose syndrome (WNS) (1), which potentially threatens >20% of all mammalian diversity (bats) (2). WNS is a deadly epidemic that has swept through the northeastern United States over the past 3 years and caused the death of >1,000,000 bats, with decreases of ≈100% in some populations (3).
This disease is associated with hibernating, cave-roosting bats. A visually conspicuous white fungus grows on the face, ears, or wings of stricken bats; infiltration of the hyphae into membranes and tissues leads to severe damage (4). Bats that exhibit WNS have little or no fat reserves, which are essential for their survival throughout and after hibernation (5). The fungus associated with WNS is a newly described, psychrophilic (cold-loving) species (Geomyces destructans) (6). It is closely related to G. pannorum, which causes skin infections in humans (7).
Although it is not known whether the fungus is primarily responsible for deaths of bats or is a secondary infection, it is directly associated with deaths of bats (5). Bacteriologic, virologic, parasitologic, and postmortem evaluations for the cause of death did not identify any other agents, which reinforces the suspicion that this fungus is the causative agent (4,5). To date, WNS has been found only in the northeastern United States. However, researchers have suggested its presence in Europe. We investigated whether G. destructans is present in bats in Europe and assessed the implications of its presence.
During intensive monitoring of bat hibernation in France, 1 bat (Myotis myotis) found on March 12, 2009, near Périgueux (45°8′N, 0°44′E), showed a powdery, white fungal growth on its nose (Figure 1, panel A), which is characteristic of WNS. Sterile dry cotton swabs were used to collect fungus material from the nose of the bat. The bat was then weighed, measured, and released. Swabs were moistened with 50 μL of sterile water and streaked onto plates containing potato dextrose agar supplemented with 0.1% mycologic peptone. Plates (9 cm in diameter) were sealed with parafilm and incubated inverted at 10°C. A dense fungus growth developed within 14 days (Figure 1, panel B).
Cultures were established by transferring inoculum to other mycologic media, including malt extract agar and Sabouraud agar. Colonies on malt extract agar were initially white but after spore production and aging they quickly darkened from the center to a dull gray, often showing a faint green hue. Spores were hyaline, irregularly curved, broadly crescent-shaped (typically 6–8 μm long and 3–4 μm wide), and narrowed at each end, one of which was broadly truncate, often showing an annular frill (Figure 1, panel C). Fungal cultures have been deposited in the culture collection of the Industrial Microbiology Department of University College Dublin (Reference IMD Z2053).
Microscopic examination of the original swab samples showed numerous spores with the above-mentioned features. The psychrophilic nature of the fungus and its species-specific morphologic features (Technical Appendix) led to the conclusion that this fungus was G. destructans, which was recently isolated from WNS-positive bats in the northeastern United States (6).
Two molecular markers were sequenced from 6 randomly chosen fungus cultures to confirm species identity. DNA was extracted by using a Blood and Tissue Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions with slight modifications (after step 3, we added an incubation time of 10 min at 70°C). The internal transcribed spacer (ITS) regions (ITS1, 5.8S, and ITS2) and the small subunit (SSU) of the rRNA gene were amplified separately.
PCRs were performed in 25-μL volumes containing 1 μL of DNA (10–75 ng/μL), 1.5 mmol/L MgCl2, 0.4 μmol/L of each primer, 0.2 mmol/L dNTP, 1× PCR buffer, and 1 U of Platinum Taq DNA Polymerase High Fidelity (Invitrogen, Carslbad, CA, USA). Identical PCR cycling conditions were used for both fragments: an initial step at 95°C for 15 s; 10 cycles at 95°C for 30 s, 60°C (reduction of 2°C every 2 cycles) for 1 min and 45 s, and 72°C for 1 min; 30 cycles at 95°C for 30 s, 50°C for 1 min and 45 s, and 72°C for 1 min; and a final step at 72°C for 10 min. PCR products were purified and sequenced in both directions by using primers listed in the Table. Complementary sequences were assembled and edited for accuracy by using CodonCode Aligner version 3.0.3 (www.codoncode.com/aligner/download.htm).
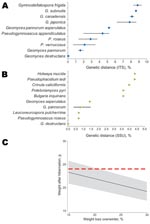
The ITS and SSU sequences from the 6 WNS fungus cultures were identical. They were deposited in GenBank as single sequences: ITS (GQ489024) and SSU (GQ489025). Sequences obtained for the 2 genetic markers showed a 100% sequence identity with the described G. destructans fungus (Figure 2, panels A, B). Thus, morphologic and genetic data support the presence of G. destructans infection in a bat in France.
Our results show that the WNS fungus was present in a bat in France and has implications for WNS research, bat conservation, and emerging infectious disease control. We suggest 3 possible scenarios for our findings. The first scenario is that the fungus has only recently arrived in Europe and all bats in Europe are now at risk for infection. Thus, conservation steps must be taken to minimize the spread of this disease, especially because this disease is specific for hibernating bats. After the hibernation period, M. myotis bats may migrate up to 436 km to reach their summer roosts (10), a behavior that could quickly increase the chance of fungus transmission. A second scenario is that the fungus has been present in Europe for a long time. Because mass deaths have not been observed in bats in Europe, these bats may be immune to WNS. Therefore, identification of mechanisms of this immunity will advance understanding of this disease and fungus resistance in mammals. The third scenario is that the G. destructans fungus is not the primary cause of death but acts as an opportunistic pathogen in bats already immunocompromised by other pathogens such as viruses or bacteria (1). Comparison of pathogens in bats in Europe and the United States infected with G. destructans should identify the primary causative agent.
The bat in our study showing fungal growth was not underweight (Figure 2, panel C), as is typical of bats in the United States with WNS (4). This finding favors the second or third scenarios. Also, a 6-year (2004–2009) annual monitoring program of wintering bat populations at the site and 5 sites within a 2-km radius did not show any cases of WNS or deaths and showed stable bat populations. The 3 scenarios indicate that studying G. destructans in bats in Europe and the United States is necessary to understand and control this disease.
Another fungus, Batrachochytrium dendrobatidis, is the etiologic agent responsible for chytridiomycosis (11), which currently threatens >50% of all amphibian species and is primarily responsible for the global decrease and extinction of >200 amphibian species in the past decade (12). Because bats account for >20% of mammalian diversity (2) and play major roles in ecosystem functions, we need to understand, monitor, and control the progression of WNS. Otherwise, we may be faced with similar unprecedented and irreversible losses of mammalian biodiversity and entire ecosystems. Because bats control insect populations throughout the world (13–15), a large decrease in bat populations would result in insect proliferations that would damage agricultural crops and spread many insect-borne diseases.
Dr Puechmaille is a postdoctoral fellow at the School of Biology and Environmental Science at University College Dublin. His research interests focus on evolution, speciation, ecology, and conservation with an emphasis on bats.
Acknowledgments
We thank Arnaud Le Houédec, Etienne Ouvrard, Les Naturalistes Vendéens/LPO-85, Christophe Hervé, Nicolas Galand, and Nicolas Harter for providing morphometric data.
This study was supported by a grant from The President of Ireland Young Researcher Award Science Foundation Ireland to E.C.T.
References
- Turner GG, Reeder DM. Update of white nose syndrome in bats, September 2009. Bat Research News. 2009;50:47–53.
- Simmons NB. Order Chiroptera. In: Wilson DE, Reeder DM, editors. Mammal species of the world: a taxonomic and geographic reference, 3rd ed. Baltimore: Johns Hopkins University Press; 2005. p. 312–529.
- Consensus statement. Austin (TX): Second WNS Emergency Science Strategy Meeting; 2009 May 27–28 [cited 2009 Nov 30]. http://www.batcon.org/pdfs/whitenose/ConsensusStatement2009.pdf
- Meteyer CU, Buckles EL, Blehert DS, Hicks AC, Green DE, Shearn-Bochsler V, Histopathologic criteria to confirm white-nose syndrome in bats. J Vet Diagn Invest. 2009;21:411–4.PubMedGoogle Scholar
- Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, Bat white-nose syndrome: an emerging fungal pathogen? Science. 2009;323:227. DOIPubMedGoogle Scholar
- Gargas A, Trest MT, Christiensen M, Volk TJ, Blehert DS. Geomyces destructans sp. nov. associated with bat white-nose syndrome. Mycotaxon. 2009;108:147–54.
- Gianni C, Caretta G, Romano C. Skin infection due to Geomyces pannorum var. pannorum. Mycoses. 2003;46:430–2. DOIPubMedGoogle Scholar
- Kunz TH, Weazen JA, Burnett CD. Changes in body mass and fat reserves in pre-hibernating little brown bats (Myotis lucifugus). Ecoscience. 1998;5:8–17.
- Johnson SA, Brack VJ, Rolley RE. Overwinter weight loss of Indiana bats (Myotis sodalis) from hibernacula subject to human visitation. Am Midl Nat. 1998;139:255–61. DOIGoogle Scholar
- Dietz C, Von Helversen O, Dietmar N. Bats of Britain, Europe and northwest Africa. London: A and C Black Publishers Ltd.; 2009.
- Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326:582–5. DOIPubMedGoogle Scholar
- Fisher MC, Garner TW, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol. 2009;63:291–310. DOIPubMedGoogle Scholar
- Williams-Guillen K, Perfecto I, Vandermeer J. Bats limit insects in a neotropical agroforestry system. Science. 2008;320:70. DOIPubMedGoogle Scholar
- Federico P, Hallam TG, McCracken GF, Purucker ST, Grant WE, Correa-Sandoval AN, Brazilian free-tailed bats as insect pest regulators in transgenic and conventional cotton crops. Ecol Appl. 2008;18:826–37. DOIPubMedGoogle Scholar
- Kalka MB, Smith AR, Kalko EK. Bats limit arthropods and herbivory in a tropical forest. Science. 2008;320:71. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 16, Number 2—February 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Emma C. Teeling, School of Biology and Environmental Science, University College Dublin, Belfield, Dublin 4, Ireland
Top