Volume 17, Number 12—December 2011
Dispatch
Continuing Threat of Influenza (H5N1) Virus Circulation in Egypt
Abstract
Reservoirs for the continuing influenza (H5N1) outbreaks in Egypt are ill-defined. Through active surveillance, we detected highly pathogenic influenza subtype H5 viruses in all poultry sectors; incidence was 5%. No other subtypes were found. Continued circulation of influenza (H5N1) viruses in various regions and poultry sectors perpetuates human exposure in Egypt.
After 150 confirmed human cases and continuous outbreaks in its different poultry production sectors, Egypt became an epicenter for highly pathogenic avian influenza (H5N1) virus activity and one of the few countries where this virus is endemic. The long-term endemicity of influenza (H5N1) virus in poultry in Egypt has generated substantial viral genetic and antigenic diversity, as has been seen in other areas (1–3), yet the ecology and epizootology of the virus in the various poultry sectors remains unknown. To determine the incidence and diversity of influenza viruses among poultry in 6 governorates in Egypt, we conducted surveillance for 1 year.
From August 2009 through July 2010, we collected 5,562 cloacal and oro-pharyngeal swab samples from poultry at 58 sites in 6 governorates in Egypt (Cairo, 4 sites, 24 birds/100,000 inhabitants; Qalubiya, 12 sites, 317,000 birds/100,000 inhabitants; Menofiya, 9 sites, 436,000 birds/100,000 inhabitants; Sharkiya, 2 sites, 375,000 birds/100,000 inhabitants; Fayyoum, 22 sites, 98,000 birds/100,000 inhabitants; and Beni Suef, 9 sites, 108,000 birds/100,000 inhabitants) (4). The selected governorates represent the main foci of the poultry industry in Egypt. In each governorate, 2–6 sites were routinely sampled monthly; samples were also collected from other sites and villages in the same governorate. Sample collection, handling, transport, screening, and subtyping by reverse transcription PCR (RT-PCR) were performed according to published protocols (5–7).
Of the cloacal swab samples, 5.0% were positive for influenza (H5N1) virus by matrix gene RT-PCR; of the oropharyngeal swab samples, 4.9% were positive (Table). All positive samples contained hemagglutinin subtype H5, determined by H5-specific RT-PCR. The percentages of positive samples by governorate were 0%–13.1% (p<0.001, Pearson χ2 test). Positivity rates were higher for governorates in the Nile Delta region (Qalubiya, Sharkiya, and Menofiya) and Cairo (6%) than for those in southern Egypt (Beni Suef and Fayyoum, 0%–3.8%).
A total of 243–764 samples were collected monthly, depending on the number of poultry available at the sites during the sample collection visit (Figure 1). At least 2 samples tested positive in every surveillance month except June 2010. The positivity rate was highest (11.1%) in October 2009. We were not able to detect a seasonal pattern of influenza outbreaks in poultry (Figure 1); however, during our surveillance period, human cases of influenza (H5N1) virus infection were reported throughout the year and peaked in January and February 2009 (8) (Figure 1).
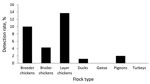
By species, ≈82% of the swab samples were collected from chickens, followed by ducks (14.7%) and other species of domestic birds (3.2%). Positivity rates differed significantly (p<0.001, Pearson χ2 test). Among chickens, 13.7% of the samples from layers, 10.0% from breeders, and 4.3% from broilers were positive. Among ducks, 1.2% of samples were positive. Only 1 pigeon swab sample was positive (Figure 2).
By collection location, the highest positivity rate (≈12%) came from poultry abattoirs (p<0.001, Pearson χ2 test). The next highest rates came from commercial farms (6.8%), followed by live-bird markets (3.3%). Only 0.9% of swab samples from backyard flocks were positive. Most (94.5%) samples were collected from apparently healthy birds; of those, 4.5% were positive. In contrast, 13.4% of samples from sick or dead birds were positive (p<0.001, Pearson χ2 test).
To identify other putative sources of human infection with influenza (H5N1) virus, we also examined a population of wild egrets (Bubulcus ibis) in urban greater Cairo. These birds congregate on trees next to the Giza Zoo in a heavy traffic area with a dense human population. RT-PCR detected influenza (H5N1) viruses in the feces of these wild egrets. Influenza (H5N1) virus shedding by the egrets threatens the exotic bird population at the zoo as well as humans in that area.
In Egypt, most swab samples positive for influenza (H5N1) virus were from chickens. Among ducks, the positivity rate was as low as 1.2%, although in other regions, ducks have been shown to play a key role in avian influenza transmission (9). All samples with positive results by RT-PCR contained highly pathogenic influenza (H5N1) viruses. The surprising lack of detection of other influenza subtypes in our surveillance may be explained by establishment of subtype H5N1 as the dominant influenza strain in poultry in Egypt. Alternatively, low-pathogenicity viruses may be circulating in different regions or different host populations not covered by our surveillance. Whatever the reason, the lack of substantial cocirculation of multiple influenza viruses reduces the chances of influenza (H5N1) virus evolution occurring in Egypt by reassortment.
In Egypt, commercial farms are major reservoirs for influenza (H5N1) virus; the positivity rate was higher for those farms (7.2%) than for backyard farms (0.9%). Because the sampled poultry at commercial farms, where biosecurity measures were generally lax, were vaccinated with commercially available subtype H5 vaccines, the effectiveness of such vaccines becomes highly questionable. The lower positivity rate among backyard poultry may be explained by the fact that the growers slaughter these birds at the first sign of disease.
Reports of influenza (H5N1) virus infections in humans in Egypt show that most of these persons had had contact with sick poultry, primarily in backyards (8,10), as has been reported in Asia (11–13). Our findings indicate that the threat to humans in Egypt is much more widespread than previously reported. We detected influenza (H5N1) viruses in poultry from all production sectors and from wild egrets in Cairo. Among specimens collected from live-bird markets and slaughterhouses in Cairo, ≈6% had positive results; these birds usually come from commercial farms in rural areas. This finding indicates that the public health concern applies not only to rural poultry growers but also to persons in urban areas.
Although we were able to detect influenza virus among poultry continuously during surveillance, we did not establish a clear seasonal pattern of outbreaks, an indicator of continuous evolution of subtype H5N1 viruses endemic to Egypt. During the same period covered by our surveillance, human cases were reported during 8 of the 12 months; incidence was highest in January and February, reflecting a seasonal pattern conforming to the climate, with influenza activity peaks in the colder months. These data suggest that the seasonality of influenza (H5N1) in humans is not explained by increased virus activity in the associated poultry population but rather by other unidentified behavioral or environmental factors.
Our surveillance findings reveal that highly pathogenic influenza (H5N1) viruses are abundant and persistent in Egypt. Closer surveillance of avian influenza viruses in domestic poultry and expansion of such surveillance to include wild and migratory birds is warranted in an effort to continuously monitor the evolution of subtype H5N1 and other influenza viruses in Egypt.
Dr Kayali is a postdoctoral research associate at St. Jude Children’s Research Hospital. His research interests are the epidemiology of influenza and viral zoonotic diseases.
Acknowledgment
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services, contract no. HHSN266200700005C, and supported by the American Lebanese Syrian Associated Charities.
References
- Balish AL, Davis CT, Saad MD, El-Sayed N, Esmat H, Tjaden JA, Antigenic and genetic diversity of highly pathogenic avian influenza A (H5N1) viruses isolated in Egypt. Avian Dis. 2010;54(Suppl):329–34. DOIPubMedGoogle Scholar
- Smith GJ, Fan XH, Wang J, Li KS, Qin K, Zhang JX, Emergence and predominance of an H5N1 influenza variant in China. Proc Natl Acad Sci U S A. 2006;103:16936–41. DOIPubMedGoogle Scholar
- Chen H, Smith GJ, Li KS, Wang J, Fan XH, Rayner JM, Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci U S A. 2006;103:2845–50. DOIPubMedGoogle Scholar
- Egyptian Ministry of Agriculture. Poultry production in Egypt. Cairo: The Ministry; 2009.
- Lee MS, Chang PC, Shien JH, Cheng MC, Shieh HK. Identification and subtyping of avian influenza viruses by reverse transcription PCR. J Virol Methods. 2001;97:13–22. DOIPubMedGoogle Scholar
- World Health Organization. WHO manual on animal influenza diagnosis and surveillance. 2nd ed. 2002 [cited 2011 Aug 1]. http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_NCS_2002.5.pdf
- Slomka MJ, Coward VJ, Banks J, Londt BZ, Brown IH, Voermans J, Identification of sensitive and specific avian influenza polymerase chain reaction methods through blind ring trials organized in the European Union. Avian Dis. 2007;51(Suppl):227–34. DOIPubMedGoogle Scholar
- Kayali G, Webby RJ, Ducatez MF, El-Sheshiny RA, Kandeil AM, Govorkova EA, The epidemiological and molecular aspects of influenza H5N1 viruses at the human–animal interface in Egypt. PLoS ONE. 2011;6:e17730. DOIPubMedGoogle Scholar
- Kim JK, Negovetich NJ, Forrest HL, Webster RG. Ducks: the “Trojan horses” of H5N1 influenza. Influenza Other Respir Viruses. 2009;3:121–8. DOIPubMedGoogle Scholar
- World Health Organization. Epidemic and pandemic alert and response. 2010 [cited 2011 Aug 1]. http://www.who.int/csr/don/archive/country/egy/en/
- Areechokchai D, Jiraphongsa C, Laosiritaworn Y, Hanshaoworakul W, O'Reilly M. Investigation of avian influenza (H5N1) outbreak in humans—Thailand, 2004. MMWR Morb Mortal Wkly Rep. 2006;55(Suppl 1):3–6.PubMedGoogle Scholar
- Dinh PN, Long HT, Tien NT, Hien NT, Mai le TQ, Phong le H, et al. Risk factors for human infection with avian influenza A H5N1, Vietnam, 2004. Emerg Infect Dis. 2006;12:1841–7. DOIPubMedGoogle Scholar
- Zhou L, Liao Q, Dong L, Huai Y, Bai T, Xiang N, Risk factors for human illness with avian influenza A (H5N1) virus infection in China. J Infect Dis. 2009;199:1726–34. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 17, Number 12—December 2011
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ghazi Kayali, Division of Virology, Department of Infectious Diseases, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, USA:
Top