Volume 17, Number 12—December 2011
Dispatch
Rickettsia parkeri in Amblyomma maculatum Ticks, North Carolina, USA, 2009–2010
Abstract
We detected Rickettsia parkeri in 20%−33% of Amblyomma maculatum ticks sampled in North Carolina. Results highlight the high frequencies of R. parkeri–infected ticks in the state with the highest annual incidence of Rocky Mountain spotted fever. Epidemiologic studies are needed to definitively link R. parkeri to cases of spotted fever rickettsiosis.
North Carolina historically reports some of the highest annual case counts of Rocky Mountain spotted fever (RMSF) and has accounted for >20% of total cases reported in the United States during the past 30 years (1–4). However, a species-specific diagnosis directly implicating infection with Rickettsia rickettsii is obtained for <10% of reported US cases. In 2010, the Centers for Disease Control and Prevention and Council of State and Territorial Epidemiologists modified the RMSF case designation to spotted fever rickettsiosis, acknowledging the complex epidemiology of tick-borne rickettsioses (5). Currently, R. parkeri is the only other tick-borne spotted fever group Rickettsia (SFGR) species known to cause disease in the southeastern United States, with >30 recognized cases from at least 9 states, including North Carolina (6). R. parkeri is detected in 20%−43% of Amblyomma maculatum ticks from the southeast, far greater than the recognized occurrence of R. rickettsii in any other tick species (6–8). We surveyed A. maculatum ticks collected from North Carolina for evidence of R. parkeri infection to assess the possibility that SFGR other than R. rickettsii result in cases categorized as RMSF in this state.
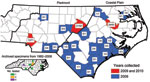
During May–September 2009 and 2010, adult ticks were collected by the Public Health Pest Management Section of the North Carolina Department of Environment and Natural Resources. Large numbers of A. maculatum ticks submitted by the general public through a tick-attachment project (www.deh.enr.state.nc.us/phpm/ticks_projects.htm) prompted further investigation in 3 counties in 2010. A total of 234 A. maculatum ticks were collected from 27 counties primarily distributed in the coastal plain and piedmont regions of North Carolina (Figure). These included 36 (2009) and 27 (2010) from the attachment project, all of which were removed from humans, except 6 (2009) that were removed from domestic dogs. The remaining 34 (2009) and 137 (2010) specimens were collected by dragging/flagging. Thirteen archived adult A. maculatum ticks collected during 1982–2008 were additionally tested, of which 6 were removed from humans. Nine adult Dermacentor variabilis ticks from the attachment project and 45 collected at sites with A. maculatum ticks were also tested. All ticks were identified by using standard taxonomic keys and were stored in 95% ethanol.
DNA was extracted by using the QIAmp DNA Mini Kit (QIAGEN, Valencia, CA, USA) for ticks collected in 2009 and Illustra Tissue and Cells genomicPrep Mini Spin Kit (GE Healthcare, Piscataway, NJ, USA) for ticks collected in 2010 and all archived A. maculatum and D. variabilis. All samples were tested by a PCR targeting a rickettsial outer membrane protein A (ompA) gene fragment. D. variabilis ticks were tested by using a broad-range SFGR-nested PCR (9). A. maculatum ticks were tested by using primers specific for R. parkeri and Candidatus Rickettsia andeanae. R. parkeri–specific primers were designed by aligning representative ompA gene sequences in GenBank for R. parkeri, R. rickettsii, R. peacockii, R. amblyommii, and Candidatus R. andeanae and identifying nonconserved regions among sequences. Primers RpompAF (5′-AATGCAGCATTTAGTGATGATGTTAA-3′) and RpompAR (5′-TCCTCCATTTATATTGCCTG-3′) were chosen. Final reagent concentrations were 300 nmol/L for each primer, 1.25 units GoTaq (Promega, Madison, WI, USA), 1.5 mmol/L MgCl2, and 200 nmol/L each dNTP. Thermal cycler conditions were as follows: 94°C (2 min); 40 cycles of 94°C (30 s), 54°C (60 s), and 72°C (90 s); and a final extension of 72°C (5 min) to amplify the 447-bp fragment. We confirmed that R. parkeri–specific primers would not amplify R. amblyommii by testing 6 A. americanum ticks infected with R. amblyommii (determined by sequencing 17-kDa antigen gene amplicon) because this species has been detected in A. maculatum ticks (10). To detect Candidatus R. andeanae, primers Rx-190-F and Rx-190-R were used in a conventional PCR (6). All rickettsial PCRs included a positive control of DNA from cultured R. parkeri (Tate’s Hell strain) or a previously confirmed Candidatus R. andeanae–infected A. maculatum tick, and water controls. DNA extractions, PCRs, and electrophoresis were performed in separate rooms or designated laboratory areas. DNA extractions from archived A. maculatum ticks were tested by PCR of a tick mitochondrial 16S rRNA gene amplicon (11) to ensure amplifiable DNA. Selected PCR products were submitted to Eurofins MWG Operon (Huntsville, AL, USA). Consensus sequences determined by ClustalX2 alignment for each sample were compared with sequences in GenBank for identification by using a BLAST search (www.ncbi.nlm.nih.gov/blast/Blast.cgi).
An additional 21 A. maculatum ticks from Mecklenburg County (2010) were processed to isolate R. parkeri as described (6). The identity of each isolate was confirmed by ompA PCR and sequence analysis.
DNA extracts from 8 female and 6 male ticks (20%) of 70 A. maculatum ticks collected in 2009 tested positive for R. parkeri (Table). Of these, 6 were collected by dragging or flagging, 2 were unattached on domestic dogs, 5 were crawling or attached on persons, and 1 was on a vehicle. Sequences from 3 R. parkeri–positive extracts from 3 different counties were 100% identical to R. parkeri; next closest in identity (98%) were R. sibirica and R. africae. In 2010, 54/164 (27 females; 27 males) (33%) A. maculatum specimens tested positive for R. parkeri. Sequences from 17 positive samples (3 from Wake County, 6 from Mecklenburg County, 8 from Martin County) were 100% identical to R. parkeri. Ten of the 2010 R. parkeri–positive ticks were attached to persons; clinical symptoms for these persons were not assessed.
Candidatus R. andeanae was detected in 9 tick extracts. Sequences of all 2010 positive ticks were 100% identical to GenBank ompA sequences for Candidatus R. andeanae. One male Candidatus R. andeanae–positive tick from Martin County was co-infected with R. parkeri, which was confirmed by sequencing. No archived A. maculatum ticks were positive by PCR for Candidatus R. andeanae or R. parkeri. However, 8 archived samples showed faintly staining bands for mitochondrial 16S rRNA gene amplicons, suggesting loss of DNA integrity in these older samples. Three isolates of R. parkeri, designated NC-3, NC-8, and NC-15, were obtained in Vero E6 cell cultures. No SFGR was detected by PCR in any D. variabilis ticks.
Until recently, A. maculatum was considered an incidental tick species in North Carolina (12,13); however, we identified an overall prevalence of R. parkeri in 29% of A. maculatum ticks from multiple sites in North Carolina considered endemic for RMSF. These data, coupled with the frequency of R. parkeri–positive ticks removed from humans, suggest that A. maculatum ticks are well established in North Carolina and that R. parkeri causes at least some cases of spotted fever rickettsiosis in this state. We examined a small number of D. variabilis ticks; however, none were infected with R. rickettsii, consistent with previous surveys of this tick for SFGR in North Carolina and other states (8,14,15). More extensive surveys of D. variabilis may be warranted to better determine the relative contribution of this tick to spotted fever rickettsiosis in North Carolina. The pathogenicity and clinical significance of Candidatus R. andeanae are unknown; however, this rickettsia is detected in A. maculatum ticks less frequently than R. parkeri and thus far has not been directly associated with human illness (6). Candidatus R. andeanae has been detected in singly infected A. maculatum ticks from Virginia (7), Florida, Mississippi, and Georgia (9) but to our knowledge has not been found in ticks co-infected with other rickettsiae. Further studies that causally link R. parkeri in A. maculatum ticks with human disease in North Carolina are necessary to incriminate it as a causative agent in this state.
Dr Varela-Stokes is an assistant professor of parasitology in the Department of Basic Sciences at Mississippi State University. Her research focuses on vector-borne diseases, particularly those caused by pathogens transmitted by tick vectors.
Acknowledgment
We thank Edward C. Swab and staff at Axiom Environmental, Inc., who collected specimens during the course of their workday and without whom the presence and impact of A. maculatum ticks in North Carolina would not have been noted. In addition, we are grateful for the assistance of Rob McHenry and Christa Rogers for contributing specimens and facilitating our collections at Cowan's Ford Wildlife Refuge. Finally, we thank Erle Chenney and Whitney Smith for their contributions to the molecular analysis of ticks.
References
- Chapman AS, Murphy SM, Demma LJ, Holman RC, Curns AT, McQuiston JH, Rocky Mountain spotted fever in the United States, 1997−2002. Vector Borne Zoonotic Dis. 2006;6:170–8. DOIPubMedGoogle Scholar
- Dalton MJ, Clarke MJ, Holman RC, Krebs JW, Fishbein DB, Olson JG, National surveillance for Rocky Mountain spotted fever, 1981−1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg. 1995;52:405–13.PubMedGoogle Scholar
- Openshaw JJ, Swerdlow DL, Krebs JW, Holman RC, Mandel E, Harvey A, Rocky mountain spotted fever in the United States, 2000−2007: interpreting contemporary increases in incidence. Am J Trop Med Hyg. 2010;83:174–82. DOIPubMedGoogle Scholar
- Treadwell TA, Holman RC, Clarke MJ, Krebs JW, Paddock CD, Childs JE. Rocky Mountain spotted fever in the United States, 1993−1996. Am J Trop Med Hyg. 2000;63:21–6.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Notice to readers: changes to the national notifiable infectious disease list and data presentation—January 2010. MMWR Morb Mortal Wkly Rep. 2010;59:11.
- Paddock CD, Fournier PE, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MG, Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl Environ Microbiol. 2010;76:2689–96. DOIPubMedGoogle Scholar
- Wright CL, Nadolny RM, Jiang J, Richards AL, Sonenshine DE, Gaff HD, Rickettsia parkeri in Gulf Coast ticks, southeastern Virginia, USA. Emerg Infect Dis. 2011;17:896–8.PubMedGoogle Scholar
- Burgdorfer W. Ecological and epidemiological considerations of Rocky Mountain spotted fever and scrub typhus. In: Walker D, editor. Biology of rickettsial diseases. Boca Raton (FL): CRC Press; 1988. p. 33−50.
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–3.PubMedGoogle Scholar
- Trout R, Steelman CD, Szalanski AL, Williamson PC. Rickettsiae in Gulf Coast ticks, Arkansas, USA. Emerg Infect Dis. 2010;16:830–2.PubMedGoogle Scholar
- Black WC IV, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodidae) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A. 1994;91:10034–8. DOIPubMedGoogle Scholar
- Apperson CS, Levine JF, Nicholson WL. Geographic occurrence of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) infesting white-tailed deer in North Carolina. J Wildl Dis. 1990;26:550–3.PubMedGoogle Scholar
- Harrison BA, Engber BR, Apperson CS. Ticks (Acari: Ixodida) uncommonly found biting humans in North Carolina. J Vector Ecol. 1997;22:6–12.PubMedGoogle Scholar
- Ammerman NC, Swanson KI, Anderson JM, Schwartz TR, Seaberg EC, Glass GE, Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg Infect Dis. 2004;10:1478–81.PubMedGoogle Scholar
- Stromdahl EY, Jiang J, Vince M, Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector Borne Zoonotic Dis. 2011;11:969–77 .DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 17, Number 12—December 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Andrea S. Varela-Stokes, Wise Center, Spring Street, Mississippi State University, Starkville, MS 39762, USA
Top