Volume 18, Number 10—October 2012
Dispatch
Human Infection with Candidatus Neoehrlichia mikurensis, China
Abstract
To identify Candidatus Neoehrlichia mikurensis infection in northeastern China, we tested blood samples from 622 febrile patients. We identified in 7 infected patients and natural foci for this bacterium. Field surveys showed that 1.6% of ticks and 3.8% of rodents collected from residences of patients were also infected.
Candidatus Neoehrlichia mikurensis was detected in 1999 in Ixodes ricinus ticks in the Netherlands and referred to as an Ehrlichia spp.–like agent (1). It was then classified as a new member of family Anaplasmataceae on the basis of ultrastructure and phylogenetic analysis (2). The agent was detected in ticks and small wild mammals in Europe and Asia (1–6) and has recently been reported to infect humans, especially immunocompromised patients in Europe (7–10). However, no cases of infection have been identified outside Europe. Moreover, the agent has not yet been isolated in pure culture, and its antigens are not available.
To investigate human infections with tick-borne agents in China, we initiated a surveillance study at Mudanjiang Forestry Central Hospital (Mudanjiang, China). This hospital is one of the largest hospitals treating patients with tick-borne infectious diseases in northeastern China, where various tick-borne agents have been detected in ticks and animal hosts (11–15).
During May 2–July 30, 2010, a total of 622 febrile patients, who had histories of recent tick bites and sought treatment at Mudanjiang Forestry Central Hospital (Figure 1) were screened for the infections of tick-borne agents. When patients were admitted, peripheral blood samples were collected and treated with EDTA. DNA as extracted by using the QIAmp DNA Blood Mini Kit (QIAGEN, Germantown, MD, USA).
For a broad-range assay, a nested PCR specific for the 16S rRNA gene (rrs) was used to detect organisms in the family Anaplasmataceae. For positive samples, 2 heminested PCRs were used to amplify the entire rrs gene. For further confirmation, a nested PCR specific for the 60-kDa heat shock protein gene (groEL) was performed. Detailed cycling conditions for all amplifications are described in the Technical Appendix.
Seven patients were found to be infected with Candidatus N. mikurensis by amplifications of the rrs and groEL genes. Amplified rrs gene (1,501 bp) and partial groEL gene (1,230 bp) sequences from these patients were identical. These sequences were also identical to genes of Candidatus N. mikurensis detected in ticks and rodents in the Asian region of Russia (5).
Serum samples were collected from patients during the acute (2–12 days after onset of illness) or convalescent (34–42 days after onset of illness) phases of illness. All samples were negative for IgG against Anaplasma phagocytophilum, Ehrlichia chaffeensis, Borrelia burgdorferi, Rickettsia heilongjiangensis, and tick-borne encephalitis virus when tested by indirect immunofluorescence assay.
All 7 patients were farmers residing in the villages in Mudanjiang. Their median age was 41 years (range 29–67 years) and 5 were men. None had been vaccinated against tick-borne encephalitis. The patients had onset of illness during May 20–July 13, 2010. The median time from the tick bite to the onset of illness and from the onset of illness to the physician visit was 8 days (range 2–35 days) and 7 days (range 1–12 days), respectively.
All patients were otherwise healthy, and none had a history of underlying immunocompromised conditions. Fever, headache, and malaise were reported for all 7 patients. Other major manifestations included nausea (5/7), vomiting (5/7), myalgia (4/7), and stiff neck (4/7). Less common symptoms were arthralgias (2/7), cough (2/7), diarrhea (1/7), confusion (1/7), and erythema (1/7). Skin erythema (multiple and oval) was seen on the neck of 1 patient.
Laboratory test results showed leukopenia in 1 patient, leukocytosis in 1 patient, thrombocytopenia in 2 patients, and anemia in 2 patients. Serum levels of alanine aminotransferase and aspartate aminotransferase were within reference ranges for all patients. Wright–Giemsa stained peripheral blood smears did not show morulae or other blood parasites.
To identify local natural foci, we performed a field investigation on infections of Candidatus N. mikurensis in ticks and rodents from areas of residences of the patients. During May–July 2010, a total of 516 host-seeking ticks, including 316 I. persulcatus, 187 Haemaphysalis concinna, and 13 Dermacentor silvarum, were collected on vegetation and individually examined. Candidatus N. mikurensis DNA was detected in 6 (1.9%) I. persulcatus and 2 (0.8%) H. concinna ticks, but no DNA was detected in D. silvarum ticks (Table).
A total of 211 rodents of various species were captured by using snap traps. After rodent species was identified, spleen specimens were collected for DNA extraction and PCR. Eight rodents of 3 species, 5 (4.6%) Clethrionomys rufocanus, 2 (5.7%) Rattus norvegicus, and 1 (33.3%) Tamias sibiricus, were positive for Candidatus N. mikurensis (Table).
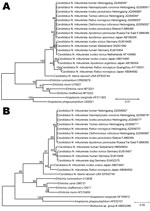
Nucleotide sequences of rrs and groEL genes of 8 ticks and 8 rodents were identical to each other and to sequences obtained from the 7 patients. Phylogenetic analysis of rrs genes showed that nucleotide sequences identified were identical to those of Candidatus N. mikurensis from Japan and the Asian region of Russia but different from sequences from Europe (99.6%–99.8% similarity) (Figure 2, panel A). Similar phylogenetic relationships were observed in a neighbor-joining tree based on groEL gene nucleotide sequences. In comparison with sequences from humans and ticks in Europe, the groEL gene sequences identified in the study showed 97.6%–98.4% similarity (Figure 2, panel B).
We have detected Candidatus N. mikurensis DNA in blood samples from 7 patients collected during the period of acute illness, which suggests that this bacterium was the etiologic agent of the infections. Our findings demonstrated human infections with Candidatus N. mikurensis in China. The rrs and groEL gene nucleotide sequences of this Candidatus N. mikurensis variant were identical to those obtained from ticks and rodents in the Asian region of Russia, which have not been reported to cause human infection.
Unlike reported cases in elderly or immunocompromised patients in whom disease developed (7–10), all 7 patients in our study had relatively mild disease. Major clinical manifestations and laboratory findings of the cases in our report, such as leukocytosis, were not similar to those of previously reported cases. It is noteworthy that the patients reported in this study were previously healthy. Thus, their clinical manifestations might be typical of Candidatus N. mikurensis infection in an otherwise healthy population. However, the number of cases in our study was limited, and clinical data were not inclusive. Clinical characteristics of Candidatus N. mikurensis infection should include detailed descriptions of additional cases.
Our finding of a Candidatus N. mikurensis variant in 1.6% of ticks and 3.8% of rodents tested suggested natural foci of the bacterium in Mudanjiang. Therefore, clinical diagnosis of Candidatus N. mikurensis infection should be considered in patients who have been exposed to areas with high rates of tick activity. It is noteworthy that Candidatus N. mikurensis was originally detected in R. norvegicus from Guangzhou Province in southeastern China (4), thereby indicating the potential threat to humans in areas other than northeastern China.
In summary, we identified Candidatus N. mikurensis as an emerging human pathogen in China. Further studies should be conducted to isolate this bacterium and investigate its epidemiologic, genetic, and pathogenic features. To guide diagnostic testing and treatment, physicians should be aware that human Candidatus N. mikurensis infections are in Heilongjiang Province and that PCR can be used as a diagnostic technique for identifying suspected infections.
Mr Li is a medical student in the State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology. His research interests include the microbiology, epidemiology, and ecology of vector-borne diseases.
Acknowledgment
This study was supported by the Natural Science Foundation of China (81130086, 81072250, and 81172729), the Chinese Basic Research Project (2010CB530201), and the Special Fund for Health Research in the Public Interest (201202019).
References
- Schouls LM, Van De Pol I, Rijpkema SG, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22.PubMedGoogle Scholar
- Kawahara M, Rikihisa Y, Isogai E, Takahashi M, Misumi H, Suto C, Ultrastructure and phylogenetic analysis of ‘Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int J Syst Evol Microbiol. 2004;54:1837–43. DOIPubMedGoogle Scholar
- Andersson M, Raberg L. Wild rodents and novel human pathogen Candidatus Neoehrlichia mikurensis, southern Sweden. Emerg Infect Dis. 2011;17:1716–8. DOIPubMedGoogle Scholar
- Pan H, Liu S, Ma Y, Tong S, Sun Y. Ehrlichia-like organism gene found in small mammals in the suburban district of Guangzhou of China. Ann N Y Acad Sci. 2003;990:107–11. DOIPubMedGoogle Scholar
- Rar VA, Livanova NN, Panov VV, Doroschenko EK, Pukhovskaya NM, Vysochina NP, Genetic diversity of Anaplasma and Ehrlichia in the Asian part of Russia. Ticks Tick Borne Dis. 2010;1:57–65. DOIPubMedGoogle Scholar
- Richter D, Matuschka FR. “Candidatus Neoehrlichia mikurensis,” Anaplasma phagocytophilum, and Lyme disease spirochetes in questing European vector ticks and in feeding ticks removed from people. J Clin Microbiol. 2012;50:943–7. DOIPubMedGoogle Scholar
- von Loewenich FD, Geissdorfer W, Disque C, Matten J, Schett G, Sakka SG, Detection of “Candidatus Neoehrlichia mikurensis” in two patients with severe febrile illnesses: evidence for a European sequence variant. J Clin Microbiol. 2010;48:2630–5. DOIPubMedGoogle Scholar
- Fehr JS, Bloemberg GV, Ritter C, Hombach M, Luscher TF, Weber R, Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg Infect Dis. 2010;16:1127–9. DOIPubMedGoogle Scholar
- Pekova S, Vydra J, Kabickova H, Frankova S, Haugvicova R, Mazal O, Candidatus Neoehrlichia mikurensis infection identified in 2 hematooncologic patients: benefit of molecular techniques for rare pathogen detection. Diagn Microbiol Infect Dis. 2011;69:266–70. DOIPubMedGoogle Scholar
- Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wenneras C. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J Clin Microbiol. 2010;48:1956–9. DOIPubMedGoogle Scholar
- Cao WC, Zhao QM, Zhang PH, Dumler JS, Zhang XT, Fang LQ, Granulocytic Ehrlichiae in Ixodes persulcatus ticks from an area in China where Lyme disease is endemic. J Clin Microbiol. 2000;38:4208–10.PubMedGoogle Scholar
- Cao WC, Zhao QM, Zhang PH, Yang H, Wu XM, Wen BH, Prevalence of Anaplasma phagocytophila and Borrelia burgdorferi in Ixodes persulcatus ticks from northeastern China. Am J Trop Med Hyg. 2003;68:547–50.PubMedGoogle Scholar
- Cao WC, Zhan L, De Vlas SJ, Wen BH, Yang H, Richardus JH, Molecular detection of spotted fever group Rickettsia in Dermacentor silvarum from a forest area of northeastern China. J Med Entomol. 2008;45:741–4. DOIPubMedGoogle Scholar
- Zhan L, Cao WC, Chu CY, Jiang BG, Zhang F, Liu W, Tick-borne agents in rodents, China, 2004–2006. Emerg Infect Dis. 2009;15:1904–8. DOIPubMedGoogle Scholar
- Zhan L, Cao WC, Jiang JF, Zhang XA, Liu YX, Wu XM, Anaplasma phagocytophilum from rodents and sheep, China. Emerg Infect Dis. 2010;16:764–8. DOIPubMedGoogle Scholar
Figures
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 18, Number 10—October 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Wu-Chun Cao, State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology, 20 Dong-Da St, Fengtai District, Beijing 100071, People’s Republic of China
Top