Volume 20, Number 1—January 2014
Dispatch
Avian Hepatitis E Virus in Chickens, Taiwan, 2013
Abstract
A previously unidentified strain of avian hepatitis E virus (aHEV) is now endemic among chickens in Taiwan. Analysis showed that the virus is 81.5%–86.5% similar to other aHEVs. In Taiwan, aHEV infection has been reported in chickens without aHEV exposure, suggesting transmission from asymptomatic cases or repeated introduction through an unknown common source(s).
Avian hepatitis E virus (aHEV) was first isolated from chickens with big liver and spleen disease or hepatitis-splenomegaly syndrome (1,2). aHEV infection in chickens can cause death and reduce egg production, resulting in economic losses in the poultry industry (3). The zoonotic characteristic of aHEV have not been verified with certainty (4); however, the virus may have public health implications related to the consumption of contaminated poultry eggs and meat, the use of poultry viscera as a culinary delicacy, and the handling of poultry.
In Taiwan, the prevalence of aHEV in avian livestock has been increasing, but the causative strain has not been known. To increase our knowledge of this growing problem, we determined the seroprevalence of aHEV antibody in chickens in Taiwan and then isolated the infecting virus and sequenced and phylogenetically analyzed its full genome to better determine the origin and evolutionary status of the virus.
In 2013, we analyzed serum samples from 1,326 chickens in 61 flocks throughout Taiwan to study the prevalence of aHEV antibodies. In addition, we collected bile samples from 150 chickens among the 4 commercial egg-layer flocks in Pingtung County, Taiwan, to isolate and identify the causative aHEV strain. All chickens appeared to be healthy and ranged in age from 30.1 to 62.8 (mean 43.9) weeks for breeders and from 19.0 to 65.1 (mean 53.1) weeks for layers (Table 1). We tested serum samples for aHEV antibodies by using an ELISA (BioChek, Reeuwijk, the Netherlands) essentially as described by the manufacturer. aHEV antibody seroprevalence was 40.57% (538/1,326) among the chickens and 95.08% (58/61) among the flocks (Table 1).
We used reverse transcription PCR to isolate the aHEV RNA genome from chicken bile, and 3 sets of degenerative primers were designed to amplify a specific region of the genome. The first and the second sets of degenerative primers were designed on the basis of multiple sequence alignments derived from the helicase gene in open reading frame (ORF) 1 and the capsid gene in ORF2 (5), respectively. Primers based on ORF1 were AHEV F-1/SD, 5′-TGTTATYACACCCACCAARACGYTG-3′ for positions 2,524–2,548; Helic R-1, 5′-CCTCRTGGACCGTWATCGACCC-3′ for positions 2,975–2,954; AHEV F-2/SD, 5′-GCCACGGCTRTTACACCYCAYGT-3′ for positions 2,573–2,595; and Helic R-2, 5′-GACCCRGGRTTCGACTGCTT-3′ for positions 2,958–2,939. Primers based on ORF2 were AHEV ORF2/F-1/SD, 5′-TCGCCYGGTAAYACWAATGC-3′ for positions 5,473–5,492; AHEV ORF2/R-1/SD, 5′-GCGTTSCCSACAGGYCGGCC-3′ for positions 5,750–5,731; AHEV ORF2/F-2/SD, 5′-ACWAATGCYAGGGTCACCCG-3′ for positions 5,485–5,504; and AHEV ORF2/R-2/SD, 5′-ATGTACTGRCCRCTSGCCGC-3′ for positions 5,726–5,707.
The third primer set was designed on the basis of 5 multiple alignments of complete or nearly complete aHEV sequences of other aHEV strains (GenBank accession nos. AM943647, GU954430, AM943646, EF206691, and AY535004) for the aligned results near the end of ORF2. The primers included nt5883/F, 5′-GGAYTATGGGAAYCAGCATG-3′ for positions 5,862–5,881; nt6579/R, 5′-ATCACAATAAATTAAACATAGGG-3′ for positions 6,600–6,578; nt6216/F, 5′-TGGGGRCCYCAGGGCGCTG-3′ for positions 6,196–6,214; and nt6498/R 5′-GAGGGGAATGTYYTACTAAG-3′ for positions 6,515–6,496.
Following the primer walking strategy, we designed sequencing primers as detailed in Table 2. We then sequenced the complete genome (6,653 bp) of the aHEV strain isolated from chickens in Taiwan (TWNaHEV; GenBank accession no. KF511797) and determined that it is 1 base pair shorter than that of the prototype aHEV (6). The cloned sequence of TWNaHEV RNA is composed of the noncoding region at the 5′ end (1–25 nt); ORF1 (26–4,618 nt), including methytransferase (191–742 nt), helicase (2,429–3,124 nt), and RNA-dependent RNA polymerase (3,167–4,618 nt); ORF3 (4,652–4,915 nt); ORF2 (4,705−6,525 nt); and the noncoding region at the 3′ end (6,525–6,653 nt).
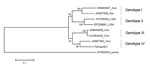
We compared the sequence of strain TWNaHEV with complete or near-complete sequences in GenBank for 7 other aHEVs that had been isolated from chickens; TWNaHEV shared 81.5%–86.5% sequence identity (GenBank accession nos. AM943647 and JN997392, respectively) with the other aHEVs. Phylogenetic analysis using the maximum likelihood method (7) with 1,000 bootstraps, based on the sequence variations in nucleotides, indicated high support for a close relationship (98%) between the aHEV genotype IV strains from Hungary and Taiwan (GenBank accession nos. JN997392 and KF511797, respectively) (Figure) (8). In addition, the aHEV genotype IV strains are close to the genotype III strains, which are represented by another strain from Hungary and a strain from China (GenBank accession nos. AM943646 and GU954430, respectively), but this relationship is supported by a bootstrap value of only 55%. Moreover, previous studies (9–13) suggested an association of genetic types of aHEV with geographic regions. In contrast, we found a mixture of strains from Taiwan, China, and Hungary (GenBank accession nos. KF511797; GU954430; and JN997392 and AM943646, respectively) classified into the separate genotype III and IV clades (Figure).
Several cases of aHEV in chickens without aHEV exposure have been reported in Taiwan. No apparent full-scale outbreaks of acute or chronic aHEV disease have occurred, yet the estimated high seroprevalence of aHEV antibodies among chickens in Taiwan indicates that the disease is now endemic. This finding suggests the possibility of aHEV transmission from asymptomatic cases or repeated introduction through an unknown common source(s). Studies on public health issues related to aHEV; the geographic prevalence and genetic diversity of aHEV; and cross-species infection with aHEV are lacking, and studies on the zoonotic properties of aHEV are incomplete but underway. Knowledge of the diffusion pattern of aHEV around Taiwan is also lacking, although it is known that horizontal, but not vertical, transmission of aHEV is possible (12,14). Given these facts, hepatitis surveillance is essential in Taiwan.
Ms Hsu is a veterinarian and completed this work as part of her master’s thesis at the School of Veterinary Medicine, National Taiwan University. Her research interests include emerging and zoonotic diseases of public health significance.
Dr Tsai is a professor in the School of Veterinary Medicine, National Taiwan University, and is director general of the Animal Health Research Institute, Council of Agriculture. His research interests are in the emerging and zoonotic diseases, especially in avian pathology and public health.
Acknowledgment
We thank several anonymous staff of Animal Health Research Institute, Council of Agriculture and North Avian Health Center, National Animal Industry Foundation, for assistance with sample collection. Our sincere appreciation is extended to X.J. Meng for his guidance on samples used to clone the avian hepatitis E virus and to M.C. Yao and J.L. Chao for partial financial support, technical instructions, and use of equipment.
References
- Payne CJ, Ellis TM, Plant SL, Gregory AR, Wilcox GE. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet Microbiol. 1999;68:119–25 and. DOIPubMedGoogle Scholar
- Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J Gen Virol. 2001;82:2449–62 .PubMedGoogle Scholar
- Morrow CJ, Samu G, Mátrai E, Klausz A, Wood AM, Richter S, Avian hepatitis E virus infection and possible associated clinical disease in broiler breeder flocks in Hungary. Avian Pathol. 2008;37:527–35 and. DOIPubMedGoogle Scholar
- Meng XJ. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol. 2010;140:256–65 and. DOIPubMedGoogle Scholar
- Sun ZF, Larsen CT, Dunlop A, Huang FF, Pierson FW, Toth TE, Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States. J Gen Virol. 2004;85:693–700 and. DOIPubMedGoogle Scholar
- Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J Gen Virol. 2004;85:1609–18 and. DOIPubMedGoogle Scholar
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9 and. DOIPubMedGoogle Scholar
- Bányai K, Toth GA, Ivanics E, Glavits R, Szentpali K, Dan A. Putative novel genotype of avian hepatitis E virus, Hungary, 2010. Emerg Infect Dis. 2012;18:1365–8 and. DOIPubMedGoogle Scholar
- Bilic I, Jaskulska B, Basic A, Morrow CJ, Hess M. Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. J Gen Virol. 2009;90:863–73 and. DOIPubMedGoogle Scholar
- Billam P, Sun ZF, Meng XJ. Analysis of the complete genomic sequence of an apparently avirulent strain of avian hepatitis E virus (avian HEV) identified major genetic differences compared with the prototype pathogenic strain of avian HEV. J Gen Virol. 2007;88:1538–44 and. DOIPubMedGoogle Scholar
- Marek A, Bilic I, Prokofieva I, Hess M. Phylogenetic analysis of avian hepatitis E virus samples from European and Australian chicken flocks supports the existence of a different genus within the Hepeviridae comprising at least three different genotypes. Vet Microbiol. 2010;145:54–61 and. DOIPubMedGoogle Scholar
- Zhao Q, Zhou EM, Dong SW, Qiu HK, Zhang L, Hu SB, Analysis of avian hepatitis E virus from chickens, China. Emerg Infect Dis. 2010;16:1469–72 and. DOIPubMedGoogle Scholar
- Smith DB, Purdy MA, Simmonds P. Genetic variability and the classification of hepatitis E virus. J Virol. 2013;87:4161–9 and. DOIPubMedGoogle Scholar
- Guo H, Zhou EM, Sun ZF, Meng XJ. Egg white from eggs of chicken infected experimentally with avian hepatitis E virus contain infectious virus, but evidence of complete vertical transmission is lacking. J Gen Virol. 2007;88:1532–7 and. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 20, Number 1—January 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hsiang-Jung Tsai, Animal Health Research Institute, Council of Agriculture, 376, Zhongzheng Road, Danshui District, New Taipei City, Taiwan 251Hsiang-Jung Tsai, Animal Health Research Institute, Council of Agriculture, 376, Zhongzheng Road, Danshui District, New Taipei City 251, TaiwanHsiang-Jung Tsai, Animal Health Research Institute, Council of Agriculture, 376, Zhongzheng Road, Danshui District, New Taipei City 251, Taiwan
Top