Volume 21, Number 12—December 2015
Dispatch
No Evidence of Gouléako and Herbert Virus Infections in Pigs, Côte d’Ivoire and Ghana
Abstract
A recent report suggested that 2 novel bunyaviruses discovered in insects in Côte d’Ivoire caused lethal disease in swine in South Korea. We conducted cell culture studies and tested serum from pigs exposed to mosquitoes in Côte d’Ivoire and Ghana and found no evidence for infection in pigs.
Orthobunyaviruses and phleboviruses are transmitted to animals and humans by blood-feeding arthropods such as mosquitoes, sandflies, and ticks (1,2). Infection can cause systemic disease, including encephalitis or hemorrhagic fevers. Members of both genera of viruses encode a nonstructural (NS) protein that suppresses the antiviral interferon response of the vertebrate host (3,4). We recently discovered 2 novel prototypic bunyaviruses in mosquitoes in Côte d’Ivoire (5,6). Named Gouléako virus (GOLV) and Herbert virus (HEBV), the viruses tentatively define 2 novel bunyavirus-family genera that are in a sister relationship to the genera Phlebovirus and Orthobunyavirus, respectively. Neither virus encodes NS proteins, nor do the viruses infect vertebrate cells or cause disease in mice that have been intracerebrally inoculated with the viruses (5–7). Replication of both viruses is blocked at temperatures above 31°C, suggesting that the viruses are unlikely to infect mammals (8).
Chung et al. recently reported that, in 2013, GOLV and HEBV caused prevalent and lethal infections in swine in South Korea (9). In that study, >500 pigs from 40 farms were tested for both viruses, and viral RNA was detected in up to 79% of diseased and 55% of healthy pigs. Dead pigs carried virus in their lungs and intestines. GOLV was isolated from swine serum in porcine kidney 15 cells. These results suggest the discovery of disease caused by these 2 novel viruses in a major livestock species. Because of the implications of this finding, we attempted verification.
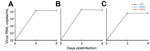
We first extended our recent cell culture studies to include porcine kidney 15 and human embryonic kidney 293 cells, which were the type of cells used by Chung et al. (9). Human hepatocellular 7 carcinoma cells were also included because they are highly susceptible to virus infection, as are Vero cells and several other cell lines we used in earlier studies (5,6). Infections with GOLV and HEBV were performed at multiplicities of infection of 1 in doublets in all cell lines. Vesicular stomatitis virus was used as a positive control at multiplicity of infection 1. Cell culture supernatants were analyzed for viral RNA after 0, 3, and 6 days by real-time reverse transcription PCR (RT-PCR) (5,6). No replication of GOLV and HEBV was detected, whereas vesicular stomatitis virus replicated to high concentrations (Figure 1). Three blind passages on fresh cells failed to yield virus.
Because cell culture experiments may not show the full host range of a specific virus, we tested serum samples collected in 2008 from Sus scrofa domestica pigs in Gouléako, the rural village where GOLV and HEBV were first isolated from mosquitoes in Côte d’Ivoire (5,6). The 28 tested samples represented nearly all the pigs kept in Gouléako at that time, all of which were constantly exposed to mosquitoes. We also tested 108 serum samples collected in 2011 from mosquito-exposed swine in Kumasi, Ghana, where mosquitoes were found to be infected with HEBV (6) and GOLV (S. Junglen, unpub. data).
All samples were tested for virus by real-time RT-PCR (5,6) and tested for antibodies against GOLV and HEBV nucleocapsid proteins by recombinant immunofluorescence assay (10). All samples were negative for the viruses (Technical Appendix Table). Technical Appendix Figure 1 shows antigen controls and results from 1 representative swine serum sample.
To compare the viruses found in pigs in South Korea with viruses found in mosquitoes in Africa, we replicated methods used by Chung et al. (9) and amplified a region of the GOLV glycoprotein precursor gene from 27 GOLV strains in mosquitoes (Technical Appendix). Nucleotide sequence distance among mosquito strains was as high as 9.0%. The viruses found in the pigs fell within the genetic diversity of viral strains of GOLV and HEBV and did not constitute phylogenetic outliers (Figure 2, panel A). The analyzed fragment had 6 aa exchanges, but they were insufficient for drawing conclusions about protein function because the fragment did not include domains putatively relevant for receptor binding (Technical Appendix Figure 2).
Small RT-PCR fragments from the RNA-dependent RNA polymerase (RdRp) gene were presented by Chung et al. for HEBV. We performed phylogenetic analyses to compare these swine-derived sequences with sequences from all mosquito-derived viruses from which we could sequence the corresponding genome region (Figure 2, panel B). Comparison of swine-derived sequences with the phylogeny of mosquito-derived HEBV strains, constructed on the basis of the third conserved region of the RdRp (Figure 2, panel C), showed that the strains from South Korea fell within the phylogenetic diversity of HEBV strains identified in West Africa. Technical Appendix Figure 3 shows nucleotide- and amino acid–based alignments.
Our results contrast with those of Chung et al. (9) for several possible reasons. First, the viruses infecting swine in South Korea may constitute variants of GOLV and HEBV that can infect vertebrates. The presence of an NSs protein in phleboviruses and orthobunyaviruses provides interferon resistance required to infect vertebrates efficiently (3,4). Because full genome sequences from swine viruses detected by Chung et al. are not available, we have no information on the presence of NS proteins in these viruses. Furthermore, our detection assays might have failed to detect variant viruses. However, our RT-PCR assays have been shown to detect variant viruses, have been validated for sensitivity (≈100 viral genome copies per mL in liquid specimens), and provide high specificity by probe detection (5,6). A concern regarding the results of Chung et al. is the use of RT-PCR assays based on SYBR Green (Thermo Fisher Scientific, Lithuania) product detection, which, from our experience, is prone to yield nonspecific results because no probe is used in this assay. Nevertheless, RT-PCR products in Chung et al. have been confirmed by sequencing. Some sequences presented by these researchers contained stop codons in the HEBV RdRp and the GOLV glycoprotein precursor genes, making it unlikely that these sequences represent replicating viruses. Besides technical explanations, these sequences could represent viral genome fragments integrated in genomes of organisms, such as insects, that are eaten by pigs in the region. Integration of RNA virids derived from flaviviruses into the host genome has been described in insects (11). Testing food eaten by swine for insect DNA or viral RNA could yield insight. In addition, we may have collected serum when no active virus infections occurred in tested animals. However, past infections would have been shown by antibody tests. Because bunyaviruses from all vertebrate-infecting genera induce antibodies against the nucleoprotein (12–14), we are confident about our choice of antigen in our assays. Chung et al. presented no serologic results to support virus detections (9).
Several technical issues in the study by Chung et al. should be clarified further. First, RNA concentration in tissue, as determined by RT-PCR, did not correlate with the success of probe-based immunohistochemistry in several organ samples (9). Second, supernatants from the virus isolate from South Korea showed high cytopathogenic activity in cell culture (103–105 cytopathogenic units/mL) but low levels of concomitant viral RNA by RT-PCR. Because no antigen detection in cells was attempted, the cytopathogenic effect could have been caused by any other virus blindly isolated. One of the most infectious and deadly swine pathogens, the porcine reproductive and respiratory syndrome virus (15), was co-detected in lung samples of dead pigs in South Korea (9).
The finding of genome fragments of GOLV and HEBV in swine in South Korea needs to be more fully explored. However, with no further independent proof of infection of swine or other vertebrates, HEBV and GOLV should not be considered epizootic pathogens or arboviruses.
Dr. Junglen is a biologist and scientist at the Institute of Virology in Bonn, Germany. Her main research interests are the diversity, evolution, and spread of arthropod-associated viruses.
Acknowledgments
We thank Pascal Trippner for technical assistance and the Max Planck Society for providing field work assistance in Côte d’Ivoire.
The project was supported by the Deutsche Forschungsgemeinschaft (grant agreement no. DR 772/12-1) to C.D.
References
- Elliott RM. Orthobunyaviruses: recent genetic and structural insights. Nat Rev Microbiol. 2014;12:673–85.DOIPubMedGoogle Scholar
- Elliott RM, Brennan B. Emerging phleboviruses. Curr Opin Virol. 2014;5:50–7. DOIPubMedGoogle Scholar
- Bridgen A, Weber F, Fazakerley JK, Elliott RM. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc Natl Acad Sci U S A. 2001;98:664–9. DOIPubMedGoogle Scholar
- Bouloy M, Janzen C, Vialat P, Khun H, Pavlovic J, Huerre M, Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J Virol. 2001;75:1371–7.file:///\\\\\\\\cdc\\\\project\\\\CCID_NCPDCID_DEISS_EIDJ\\\\EID%20Production\\\\Editorial\\\\EDITING\\\\Rhonda\\\\December\\\\D-14-1840\\\\%20PubMedDOIPubMedGoogle Scholar
- Marklewitz M, Handrick S, Grasse W, Kurth A, Lukashev A, Drosten C, Gouléako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. J Virol. 2011;85:9227–34.DOIPubMedGoogle Scholar
- Marklewitz M, Zirkel F, Rwego IB, Heidemann H, Trippner P, Kurth A, Discovery of a unique novel clade of mosquito-associated bunyaviruses. J Virol. 2013;87:12850–65. DOIPubMedGoogle Scholar
- Auguste AJ, Carrington CV, Forrester NL, Popov VL, Guzman H, Widen SG, Characterization of a novel Negevirus and a novel Bunyavirus isolated from Culex (Culex) declarator mosquitoes in Trinidad. J Gen Virol. 2014;95:481–5. DOIPubMedGoogle Scholar
- Marklewitz M, Zirkel F, Kurth A, Drosten C, Junglen S. Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proc Natl Acad Sci U S A. 2015;112:7536–41.DOIPubMedGoogle Scholar
- Chung HC, Nguyen VG, Goede D, Park CH, Kim AR, Moon HJ, Gouléako and Herbert viruses in pigs, Republic of Korea, 2013. Emerg Infect Dis. 2014;20:2072–5. DOIPubMedGoogle Scholar
- Meyer B, Muller MA, Corman VM, Reusken CB, Ritz D, Godeke GJ, Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20:552–9. DOIPubMedGoogle Scholar
- Crochu S, Cook S, Attoui H, Charrel RN, De Chesse R, Belhouchet M, Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J Gen Virol. 2004;85:1971–80. DOIPubMedGoogle Scholar
- Lazutka J, Zvirbliene A, Dalgediene I, Petraityte-Burneikiene R, Spakova A, Sereika V, Generation of recombinant Schmallenberg virus nucleocapsid protein in yeast and development of virus-specific monoclonal antibodies. J Immunol Res. 2014;2014:160316. . Epub 2014 May 29. DOIPubMedGoogle Scholar
- Ergunay K, Kocak Tufan Z, Bulut C, Kinikli S, Demiroz AP, Ozkul A. Antibody responses and viral load in patients with Crimean-Congo hemorrhagic fever: a comprehensive analysis during the early stages of the infection. Diagn Microbiol Infect Dis. 2014;79:31–6. DOIPubMedGoogle Scholar
- Williams R, Ellis CE, Smith SJ, Potgieter CA, Wallace D, Mareledwane VE, Validation of an IgM antibody capture ELISA based on a recombinant nucleoprotein for identification of domestic ruminants infected with Rift Valley fever virus. J Virol Methods. 2011;177:140–6. DOIPubMedGoogle Scholar
- Chand RJ, Trible BR, Rowland RR. Pathogenesis of porcine reproductive and respiratory syndrome virus. Curr Opin Virol. 2012;2:256–63. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 21, Number 12—December 2015
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Sandra Junglen, Institute of Virology, University of Bonn Medical Center, Sigmund-Freud Str 25, 53127 Bonn, Germany
Top