Volume 26, Number 3—March 2020
Research
Pregnancy Outcomes among Women Receiving rVSVΔ-ZEBOV-GP Ebola Vaccine during the Sierra Leone Trial to Introduce a Vaccine against Ebola
Figure 1
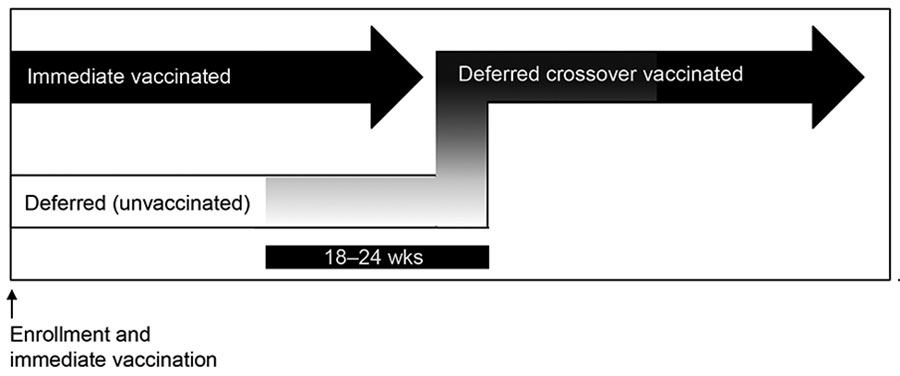
Figure 1. Enrollment and vaccination period for 84 participants in Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE). Three participants randomized to the immediate group were unvaccinated. After vaccination, participants in the deferred group were eligible for vaccination at 18–24 weeks postenrollment. Upon vaccination, participants in the deferred group were referred to as the deferred crossover vaccinated group.
1These first authors contributed equally to this article.
2These authors were co–principal investigators.
Page created: February 20, 2020
Page updated: February 20, 2020
Page reviewed: February 20, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.