Volume 27, Number 12—December 2021
Research Letter
Borrelia miyamotoi in Human-Biting Ticks, United States, 2013–2019
Abstract
During 2013–2019, Borrelia miyamotoi infection was detected in 19 US states. Infection rate was 0.5%–3.2%; of B. miyamotoi–positive ticks, 59.09% had concurrent infections. B. miyamotoi is homogeneous with 1 genotype from Ixodes scapularis ticks in northeastern and midwestern states and 1 from I. pacificus in western states.
Borrelia miyamotoi, a relapsing fever group spirochete (1), was first isolated from Ixodes persulcatus ticks in Japan in 1995 (2) and later detected in Ixodes ticks in the United States and Europe (3–5). Although B. miyamotoi bacteria have been mainly detected in I. ricinus species complex ticks that transmit B. burgdorferi worldwide, the vector specificity needs further study because investigators have found B. miyamotoi in multiple tick species (6). B. miyamotoi has 3 geographically distinct genotypes: Asian, European, and American. In the United States, B. miyamotoi bacteria have been found in field-collected I. scapularis ticks in the northeastern and northern midwestern regions, where the average infection rate is 1.9% (7). However, an expanded geographic study of the prevalence of B. miyamotoi in human-biting ticks, its genotypes, and concurrent infections with other tickborne pathogens is warranted.
Human-biting ticks were submitted to the public tick testing program at the University of Massachusetts (Amherst, Massachusetts, USA) during May 2013–December 2019. We extracted DNA from individual ticks using the Epicenter Master Complete DNA and RNA Purification Kits (Lucigen, https://www.lucigen.com). We performed a species-specific quantitative PCR (qPCR) for differentiation of I. scapularis and I. pacificus ticks (8). To detect Borrelia bacteria, we first applied a genus-specific detection assay, followed by specific qPCR assays for B. burgdorferi sensu lato and B. miyamotoi. We detected the tickborne pathogens Anaplasma phagocytophilum, Babesia microti, B. mayonii, and Ehrlichia muris–like agent (EMLA) by a multiplex qPCR assay targeting different genes. We used a qPCR assay targeting tick 16S mtDNA gene as an internal control (8). We sequenced 3 partial gene fragments, 16S rDNA (16S) (9), flagellin (fla) (6), and glycerophosphodiester phosphodiesterase (glpQ) (6), for B. miyamotoi samples that were positive by qPCR.
We received and tested 39,198 ticks found on humans for B. miyamotoi during May 2013–December 2019. Of those, 38,855 (99.12%) ticks originated from the continental United States, comprising 18 tick species (Table). Although Ixodes ticks are the main vectors for B. miyamotoi, we did not detect B. miyamotoi DNA in I. affinis, I. angustus, I. cookei, I. dentatus, I. marxi. I. muris, or I. spinipalpis ticks. We detected B. miyamotoi in I. pacificus (14/1,497, 0.94%) and I. scapularis (594/34,621, 1.72%) ticks.
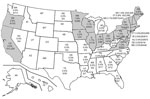
B. miyamotoi was found in 19 states; infection rates were 0.5%–3.2% (Figure). In the western United States, B. miyamotoi was found in I. pacificus ticks in Oregon and California (14/1,497, 0.94%). Although I. scapularis ticks are distributed across the eastern United States, no B. miyamotoi–positive ticks were detected south of Virginia. B. miyamotoi–positive ticks were concentrated in the Northeast and upper Midwest (594 of 34,621, 1.72%) (Figure). Lyme disease remains the principal public health concern; the causative agent, B. burgdorferi (11,287/34,621; 32.60%, 95% CI 32.1%–33.1%), was 19 times more prevalent than B. miyamotoi (594/34,621, 1.72%) in I. scapularis ticks.
On average, prevalence of B. miyamotoi infection in I. scapularis ticks (1.72%, 95% CI 1.58%–1.86%) was higher than in I. pacificus ticks (0.94%, 95% CI 0.51%–1.56%). The prevalence of B. miyamotoi in I. pacificus ticks was 1.00% (95% CI 0.53%–1.7%) in adults (13/1,300), 0.53% (95% CI 0.01%–2.9%) in nymphs (1/190), and 0.00% (95% CI 0%–40.1%) in larvae (0/7). The prevalence of B. miyamotoi in I. scapularis ticks was 1.80% (95% CI 1.64%–1.97%) in adults (456/25,376), 1.54% (95% CI 1.29−1.83%) in nymphs (133/8,615), and 0.79% (95% CI 0.26%–1.84%) in larvae (5/630).
Of 594 B. miyamotoi–positive I. scapularis ticks, 351 (59.09%) had concurrent infections. We found 293 (49.33%) I. scapularis ticks had a dual infection with B. miyamotoi: 220 (37.04%) were also infected with B. burgdorferi s.l., 43 (7.24%) with A. phagocytophilum, and 30 (5.05%) with B. microti. We further found 52 (8.75%) had a triple infection with B. miyamotoi: 23 (3.87%) were also infected with B. burgdorferi s.l. and A. phagocytophilum, 22 (3.70%) with B. burgdorferi s.l. and B. microti, and 7 (1.18%) with A. phagocytophilum and B. microti. Six (1.01%) of the B. miyamotoi–positive ticks had a quadruple infection with B. miyamotoi, B. burgdorferi s.l., A. phagocytophilum, and B. microti. No ticks with B. mayonii or EMLA were additionally infected with B. miyamotoi.
Multilocus sequence typing of the 16S, fla, and glpQ genes revealed 2 distinct B. miyamotoi genotypes separated by their tick vectors, I. scapularis ticks in the Northeast and upper Midwest and I. pacificus ticks in the West (Appendix). Whereas the 16S gene sequences were identical among all isolates, variable sites were found among fla and glpQ nucleotide sequences. Among 14 I. pacificus tick–borne B. miyamotoi isolates, all fla and glpQ sequences were identical. A previously reported A/G substitution in B. miyamotoi fla sequences from I. pacificus ticks (5,9) was outside of our sequenced fla fragment (Appendix). The genetic identity between the 2 tick species–specific genotypes was 0.996 for fla and 0.986 for glpQ. Unlike heterogeneous B. burgdorferi populations, B. miyamotoi appears to be very homogeneous within its respective tick vectors.
Dr. Xu is a research professor in the department of microbiology, University of Massachusetts–Amherst. His research interests include ticks and tickborne diseases.
References
- Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–9. DOIPubMedGoogle Scholar
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, et al. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int J Syst Bacteriol. 1995;45:804–10. DOIPubMedGoogle Scholar
- Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. DOIPubMedGoogle Scholar
- Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour AG. Typing of Borrelia relapsing fever group strains. Emerg Infect Dis. 2004;10:1661–4. DOIPubMedGoogle Scholar
- Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43:120–3. DOIPubMedGoogle Scholar
- Jiang BG, Jia N, Jiang JF, Zheng YC, Chu YL, Jiang RR, et al. Borrelia miyamotoi infections in humans and ticks, northeastern China. Emerg Infect Dis. 2018;24:236–41. DOIPubMedGoogle Scholar
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, et al. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120–31. DOIPubMedGoogle Scholar
- Xu G, Pearson P, Dykstra E, Andrews ES, Rich SM. Human-biting Ixodes ticks and pathogen prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis. 2019;19:106–14. DOIPubMedGoogle Scholar
- Cook VJ, Fedorova N, Macdonald WP, Lane RS, Barbour AG. Unique strain of Borrelia miyamotoi in Ixodes pacificus ticks, California, USA. Emerg Infect Dis. 2016;22:2205–7. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: November 04, 2021
Table of Contents – Volume 27, Number 12—December 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Guang Xu, University of Massachusetts—Microbiology, Fernald Hall Room B1, 270 Stockbridge Rd, University of Massachusetts, Amherst, MA 01003, USA
Top