Volume 30, Number 10—October 2024
Dispatch
Autochthonous Human Babesia divergens Infection, England
Abstract
We describe a case of autochthonous human Babesia divergens infection in an immunocompetent woman in England. The patient had fever, hemolysis, multiorgan failure, and 18% parasitemia. We confirmed B. divergens by 18S rDNA PCR and sequencing. Clinicians should consider babesiosis as a differential diagnosis in patients with unexplained hemolysis.
Babesiosis is caused by intraerythrocytic protozoa from the genus Babesia. First reported in 1957 (1), babesiosis is now described worldwide. Babesia infecting humans come from 4 clades (2): 3 clades of small Babesia, 1 including B. microti, which exists as a species complex; 1 including B. duncani; and 1 including B. divergens, which, despite being small, is related to the 1 clade of large Babesia spp., which infects ungulates. Ixodes spp. ticks transmit Babesia between animal hosts. Each Babesia–vector–mammal host system has its own characteristics, and the ecology and bionomics of the vector tick define the pattern of risk for the human population. Humans are accidental hosts and can also acquire babesiosis by horizontal transmission in blood products and, in rare instances, via transplacental transmission (3).
Most human babesiosis cases are caused by B. microti species complex or B. divergens, but as recognition of human cases increases, other species, some newly described, have been found in humans. B. microti is endemic in the Northeast and northern Midwest United States, and ≈2,000 human B. microti babesiosis cases are reported annually (4). However, cases of B. divergens infections are rare, ≈50 reports in the literature, and often cause more severe illness (5).
In the United Kingdom, increasing Babesia spp. prevalence in Ixodes ticks has been noted (6), but only 1 human case of B. divergens babesiosis has been reported, from Scotland in 1979 (7). We describe a case of autochthonous B. divergens infection in England.
A 72-year-old retired nurse was admitted to a hospital in southwest England after 4 days of fever, body aches, loin pain, and frank hemoglobinuria. She received ciprofloxacin in primary care the preceding day for presumed urinary tract infection, but vomiting and jaundice subsequently developed. Physical examination confirmed fever (>40°C), tachycardia, and jaundice, but no other findings. Hemoglobin was 75 g/L and bilirubin 190 μmol/L.
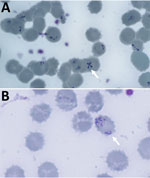
Blood film showed intraerythrocytic parasites with the Maltese cross formation, pathognomonic for Babesia spp. (Figure 1). We published that morphology as an update to raise awareness in hematology laboratories (8). Peripheral parasitemia in erythrocytes was 18% at diagnosis (Table). After consulting the Hospital for Tropical Diseases (HTD), we began treatment with intravenous clindamycin (600 mg every 6 h) and quinine (10 mg/kg every 8 h). One day into admission the patient deteriorated, had severe hypoxia requiring intubation, and was transferred by helicopter to the HTD intensive care unit (ICU).
The patient had no underlying immunosuppressive conditions and no history of splenectomy or reduced splenic function. She lived in a coastal town in Devon, UK; her only travel abroad in the preceding 12 months was a vacation in Tenerife, Spain, 5 months earlier. In the weeks before admission, she took walks along the coast, where cattle (the mammal host of B. divergens) grazed. Although ticks were in the area, she had not noticed any bites. She had no companion animals, but her daughter had a dog. She had never received blood products.
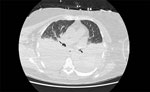
The patient’s illness was complicated by anuric acute kidney injury and fluid-refractory hypotension requiring renal dialysis and vasopressor support. Bilateral exudative pleural effusions developed (Figure 2), and she had hospital-acquired pneumonia. Severe intravascular hemolysis and black urine continued (Figure 3), which later showed evidence of hypersplenism, extravascular hemolysis, and thrombocytopenia. Her direct antiglobulin test remained negative throughout hospitalization. She underwent manual exchange transfusion with 4 units of O-negative blood and subsequent transfusion with 2 units of M- and S-antigen–negative erythrocytes on day 9 (Table). Because her recovery was protracted and thrombocytopenia was evolving, we added intravenous doxycycline (200 mg every 24 h) for possible Anaplasma co-infection.
Despite good parasitological response to treatment, the patient suffered a prolonged period of encephalopathy during convalescence. Magnetic resonance imaging of the brain showed an old cerebellar infarct, cerebrospinal fluid analysis was unremarkable, and an electroencephalogram showed nonspecific cerebral dysfunction.
Results of testing for immunoglobulins, complement blood tests, lymphocyte subsets, nuclear antibodies, tissue-transglutaminase antibodies, pneumococcal antibodies, and serum protein electrophoresis were all within reference ranges. Results of HIV serology and markers for hepatitis B and C viruses were negative; abdominal ultrasonography ruled out anatomic hyposplenia.
The patient’s encephalopathy gradually resolved, and she was extubated on day 13 of her ICU stay. Blood films on day 14 of treatment were negative, and antiparasitic agents were discontinued 24 hours later (Table). She subsequently recovered renal function and no longer required dialysis.
B. divergens infection was confirmed by 18S PCR and genomic sequencing from a blood sample drawn shortly after ICU admission (Appendix). Acute-phase serology was negative for tickborne encephalitis, Rickettsia, Borrelia, and Anaplasma. Convalescent serology for Anaplasma gave a weak, nonspecific reaction.
B. divergens, transmitted by Ixodes ricinus, has been in England, including Devon, for >100 years (9). In mild bovine cases, babesiosis (also called Redwater fever) causes fever and anorexia; severe cases result in fatal hemolytic anemia with hemoglobinuria. This case documents emergence of autochthonous human babesiosis in England. Public health conducted a tick survey in the patient’s local area but found no ticks carrying Babesia spp. (10). Serologic surveys were not possible because B. divergens serology is unavailable in the United Kingdom; whether subclinical human B. divergens infections occurred in the locality at the time is unknown, but clinicians and veterinarians in England were notified of the case to raise awareness.
This case highlights the potential for severe B. divergens infection in the absence of depressed immunity. Severe B. divergens infection causes influenza-like illness and hemolysis, after which ≈40% of patients have multiorgan failure and die (5). In a case series in Europe, 84% of B. divergens infections were in patients with previous splenectomy (3). However, PCR-confirmed B. divergens infection was found in an immunocompetent adult in France, demonstrating the parasite’s ability to cause illness in previously healthy persons (11), as in our case. We considered whether Anaplasma co-infection increased illness severity in our patient, but that was not proven. For B. microti, patients with B. burgdorferi co-infection reportedly have more symptoms and longer illness than patients with either infection alone, although no uniform agreement exists between studies on co-infection, neither in humans nor animal models (12). Nevertheless, dual infections are increasingly seen, as have triple diagnoses with babesiosis, Lyme disease, and anaplasmosis (13). Thus, clinicians should suspect multi-infection in patients with an initial babesiosis diagnosis.
Clinical laboratories diagnose B. divergens via blood film identification and PCR confirmation (13). Babesiosis treatment options include oral atovaquone and azithromycin in mild disease or intravenous clindamycin and quinine in severe cases (13). Extrapolating from B. microti treatment, oral atovaquone plus intravenous azithromycin is an option in B. divergens cases, but no randomized controlled trials or pharmacokinetic studies on B. divergens in humans are available. Exchange transfusion is recommended for parasitemia >10%, or moderate parasitemia with severe hemolytic anemia or organ dysfunction (13), and novel approaches to exchange transfusion have been suggested (14). No published trials are available, but to reduce parasitic invasion of additional erythrocytes in our patient, we administered 2 units of M- and S-antigen–negative erythrocytes, which are resistant to B. divergens invasion, during the second transfusion (14).
In summary, the clinical and laboratory features of babesiosis and its rarity could lead clinicians to misdiagnose babesiosis as leptospirosis, urosepsis, or biliary sepsis and thus cause delays in appropriate therapy. Babesia also can be morphologically misidentified as Plasmodium. Furthermore, Borrelia, Anaplasma, or Ehrlichia co-infection can complicate the illness. Physicians should consider babesiosis as a differential diagnosis in patients with unexplained intravascular hemolysis, especially in rural areas.
Dr. Zabala is a senior clinical fellow in Infectious Diseases and Internal Medicine at University College Hospital in London, UK, and a research scientist at the Infectious Diseases Data Observatory in Oxford, UK. His research interests are emerging infectious diseases and the pharmacoepidemiology of poor-quality antibiotics.
References
- Skrabalo Z, Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop. 1957;9:11–6.PubMedGoogle Scholar
- Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. Int J Parasitol. 2000;30:1323–37. DOIPubMedGoogle Scholar
- Hildebrandt A, Gray JS, Hunfeld KP. Human babesiosis in Europe: what clinicians need to know. Infection. 2013;41:1057–72. DOIPubMedGoogle Scholar
- Abdullah S, Helps C, Tasker S, Newbury H, Wall R. Prevalence and distribution of Borrelia and Babesia species in ticks feeding on dogs in the U.K. Med Vet Entomol. 2018;32:14–22. DOIPubMedGoogle Scholar
- Entrican JH, Williams H, Cook IA, Lancaster WM, Clark JC, Joyner LP, et al. Babesiosis in man: report of a case from Scotland with observations on the infecting strain. J Infect. 1979;1:227–34. DOIGoogle Scholar
- Chan WY, MacDonald C, Keenan A, Xu K, Bain BJ, Chiodini PL. Severe babesiosis due to Babesia divergens acquired in the United Kingdom. Am J Hematol. 2021;96:889–90. DOIPubMedGoogle Scholar
- McFadzean H, Johnson N, Phipps LP, Swinson V, Boden LA. Surveillance and risk analysis for bovine babesiosis in England and Wales to inform disease distribution. Animals (Basel). 2023;13:2118. DOIPubMedGoogle Scholar
- Johnson N, Phipps P, Godbole G, Hansford K, Johnston C, White M, et al. Preventing tick exposure in vets and farmers. Vet Rec. 2020;187:195. DOIPubMedGoogle Scholar
- Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, et al. Babesiosis in immunocompetent patients, Europe. Emerg Infect Dis. 2011;17:114–6. DOIPubMedGoogle Scholar
- Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T, et al.; Deer-Associated Infection Study Group. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184–91. DOIPubMedGoogle Scholar
- Smith RP, Hunfeld K-P, Krause PJ. Management strategies for human babesiosis. Expert Rev Anti Infect Ther. 2020;18:625–36. DOIPubMedGoogle Scholar
- Jajosky RP, Jajosky AN, Jajosky PG. Optimizing exchange transfusion for patients with severe Babesia divergens babesiosis: Therapeutically-Rational Exchange (T-REX) of M antigen-negative and/or S antigen-negative red blood cells should be evaluated now. Transfus Clin Biol. 2019;26:76–9. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: September 19, 2024
Table of Contents – Volume 30, Number 10—October 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Peter L. Chiodini, UKHSA Malaria Reference Laboratory, The London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK
Top