Volume 30, Number 11—November 2024
Research Letter
Influenza A(H5N1) Virus Resilience in Milk after Thermal Inactivation
Figure 1
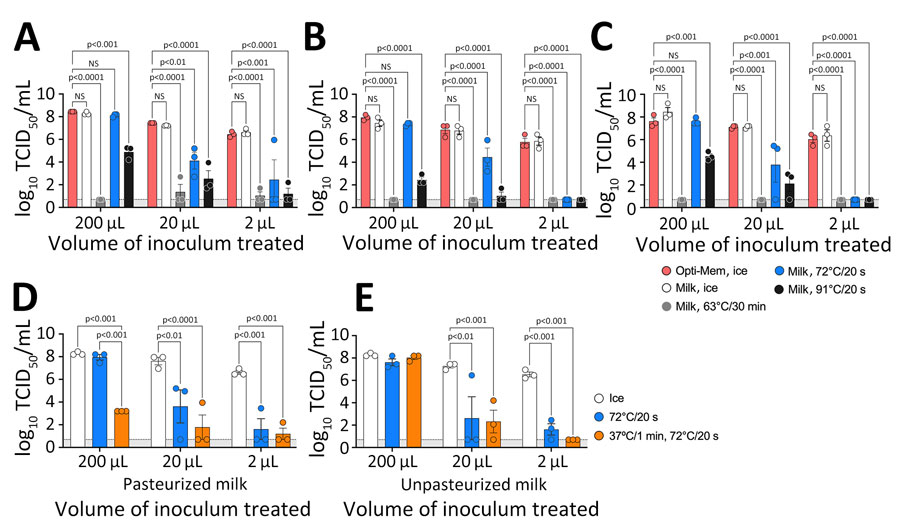
Figure 1. Heat treatment of influenza virus in milk. A–C) We diluted influenza A viruses in Opti-Mem control media (Fisher Scientific, https://www.fishersci.com) or commercial off-the-shelf pasteurized whole milk and heat-treated samples of different volumes at the times and temperatures shown; we calculated time from the moment the sample was placed in the heat block. A sandwich design in a heat block ensured uniform temperature exposure. After treatment, we chilled samples on ice for 5 minutes, adjusted them to a final volume of 200 μL, and titrated by TCID50 in MDCK cells (10). Results are shown for reverse genetics wild-type strain A/Puerto Rico/8/1934 (H1N1) (A); Vietnam/1203/04, a reverse genetics virus carrying the H5 hemagglutinin and N1 neuraminidase segments from A/Vietnam/1203/2004 (H5N1) in the background of PR/8/34, with the H5 segment modified with a monobasic cleavage site (Δ) (B); and a field isolate of the wild-type highly pathogenic strain A/turkey/Indiana/3707-003/2022 (H5N1) (C). D, E) A/Puerto Rico/8/1934 (H1N1) strain was spiked on pasteurized (D) and unpasteurized (E) milk samples at the times and temperatures shown. Circles indicate individual measurements; error bars indicate 95% CIs. Light gray shaded area indicates log10 TCID50 value of 1. NS, not significant; TCID50, 50% tissue culture infectious dose.
References
- Ly H. Highly pathogenic avian influenza H5N1 virus infections of dairy cattle and livestock handlers in the United States of America. Virulence. 2024;15:
2343931 . DOIPubMedGoogle Scholar - Garg S, Reed C, Davis CT, Uyeki TM, Behravesh CB, Kniss K, et al. Outbreak of highly pathogenic avian influenza A(H5N1) viruses in US dairy cattle and detection of two human cases—United States, 2024. MMWR Morb Mortal Wkly Rep. 2024;73:501–5. DOIPubMedGoogle Scholar
- Cohen J, Enserink M. Bird flu appears entrenched in U.S. dairy herds. Science. 2024;384:493–4. DOIPubMedGoogle Scholar
- US Food and Drug Administration. 2019. Grade A pasteurized milk ordinance [cited 2024 Aug 28]. https://www.fda.gov/food/milk-guidance-documents-regulatory-information/national-conference-interstate-milk-shipments-ncims-model-documents
- Guan L, Eisfeld AJ, Pattinson D, Gu C, Biswas A, Maemura T, et al. Cow’s milk containing avian influenza A(H5N1) virus—heat inactivation and infectivity in mice. N Engl J Med. 2024;391:87–90. DOIPubMedGoogle Scholar
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–7. DOIGoogle Scholar
- Tomasula PM, Kozempel MF, Konstance RP, Gregg D, Boettcher S, Baxt B, et al. Thermal inactivation of foot-and-mouth disease virus in milk using high-temperature, short-time pasteurization. J Dairy Sci. 2007;90:3202–11. DOIPubMedGoogle Scholar
- Kaiser F, Morris DH, Wickenhagen A, Mukesh R, Gallogly S, Yinda KC, et al. Inactivation of avian influenza A(H5N1) virus in raw milk at 63°C and 72°C. N Engl J Med. 2024;391:90–2. DOIPubMedGoogle Scholar
- Spackman E, Anderson N, Walker S, Suarez DL, Jones DR, McCoig A, et al. Inactivation of highly pathogenic avian influenza virus with high temperature short time continuous flow pasteurization and virus detection in bulk milk tanks. J Food Prot. 2024;87:
100349 . DOIPubMedGoogle Scholar - World Health Organization, Global Influenza Program, Global Influenza Surveillance and Response System. Manual for the laboratory diagnosis and virological surveillance of influenza: 2011 [cited 2024 May 20]. https://www.who.int/publications/i/item/manual-for-the-laboratory-diagnosis-and-virological-surveillance-of-influenza