Volume 30, Number 9—September 2024
Synopsis
Morphologic and Molecular Identification of Human Ocular Infection Caused by Pelecitus Nematodes, Thailand
Abstract
Nematodes of the Onchocercidae family, such as Pelecitus spp., are filarial parasites of medical and veterinary importance. Although infections are widely distributed among avian species, only 2 cases of human Pelecitus ocular infection, both in South America, have been reported. We describe a 61-year-old man in northeast Thailand diagnosed with an ocular infection. Morphologic characteristics suggested the causative agent was a female Pelecitus nematode: coiled body, rounded anterior and posterior extremities, a distinct preesophageal cuticular ring, lateral alae, a postdeirid, and a protuberant vulva. Sequences of the 12S rDNA gene indicated 95%–96% identity and cox1 gene 92%–96% identity with published P. copsychi sequences. P-distance for cox1 sequences between the causative agent and P. copsychi was 6.71%. Phylogenetic trees of 12S rDNA and cox1 genes indicated the species differed from but is closely associated with P. copsychi. Healthcare providers should be aware of the threat of ocular infection from Pelecitus spp. nematodes.
Ocular parasitosis is relatively rare, and causative agents vary by geographic area (1). Manifestations vary according to the parasite’s location. A live parasite in the anterior chamber of the eye can lead to anterior uveitis or secondary glaucoma. Various parasites, such as representatives of the genera Gnathostoma, Onchocerca, and Angiostrongylus, have been reported in the literature to cause similar conditions (1). In Southeast Asia, ocular gnathostomiasis and angiostrongyliasis often manifest with a live parasite in the anterior chamber (2,3).
The nematode genus Pelecitus belongs to the Onchocercidae family, which includes filariae of medical and veterinary importance. Among the 21 species of Pelecitus nematodes, 18 are found in birds and 3 in mammals (4,5), most distributed in Africa and South America (4). Birds serve as definitive hosts or reservoirs. Pelecitus spp. nematodes are transmitted by blood-sucking arthropods, such as mosquitoes, chewing lice, and tabanids (6).
Within the Indomalayan realm, P. ceylonensis, P. galli, and P. copsychi nematodes have been identified in animal hosts in Sri Lanka and Malaysia (5,7,8). In humans, 2 cases of Pelecitus infection have been discovered in Colombia and Brazil (9,10). However, in both reports, the parasites were identified on the basis of morphologic characteristics only.
In this study, we identified the causative agent of intraocular infection in a patient outside South America as a nematode species of the genus Pelecitus. We subsequently corroborated the preliminary identification based on morphologic characteristics using molecular studies of the mitochondrial 12S ribosomal RNA and the cytochrome c oxidase subunit 1 (cox1) genes (11,12). This study provides a morphologic description and details concerning the phylogenetic position of the Pelecitus sp. nematode identified in this article. Our case report was approved by the ethics committee of Mahasarakham University (approval no. 181-200/2023).
Case Report
An otherwise healthy 61-year-old man in Thailand sought treatment for gradually increasing eye pain, redness, light sensitivity, and slight vision loss in his left eye. Symptoms persisted for ≈1 month. He was a farmer in northeastern Thailand and had not traveled outside the country. He regularly consumed traditional dishes containing raw and partially cooked ingredients, including beef and fish. He was diagnosed with an intraocular parasite at a private clinic, where staff performed an Nd:YAG (neodymium-doped yttrium aluminum garnet) laser procedure to immobilize the parasite before the patient was referred to the hospital. We postulate that the infection in this patient might have resulted from a bite from a hematophagous arthropod.
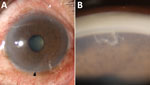
Upon examination, the patient had best-corrected visual acuity of 20/20 in the right eye and 20/40 in the left eye. Intraocular pressure measurements were 18 mm Hg in the right eye and 14 mm Hg in the left. The right eye examination was unremarkable, but the left eye showed ciliary injection (Figure 1, panel A) and grade 1+ anterior chamber cells. A live, moving parasite was observed in the inferior anterior chamber, more distinctly during gonioscopic examination (Figure 1, panel B). The posterior segment was within reference limits. Complete blood count results showed leukocytosis of 12,640 cells/mm3 and eosinophilia of 5.5% (absolute eosinophil count 695.2 cells/mm3). Fecal examination did not reveal parasites or eggs.
We performed urgent surgical removal to prevent posterior or extraocular migration. After the operation, we treated the patient with prednisolone acetate 1% eye drops (every 2 h), moxifloxacin 0.5% eye drops (4×/d), and oral albendazole (400 mg 2×/d for 5 d). At 1-month follow-up, the patient returned with a reduction in pain and redness. The best-corrected visual acuity was 20/30 and the intraocular pressure 10 mm Hg in the left eye. We found no active inflammation upon slit-lamp examination. Prednisolone acetate 1% eye drops were gradually tapered. At the 1-year follow-up, the patient was doing well without recurrence of ocular symptoms. We did not perform a blood test at follow-up.
Morphologic Identification of the Parasite
We fixed the causative agent in 70% ethanol and subsequently cleared it in lactophenol (R & M Chemicals, https://www.evergreensel.com.my) for morphologic examination under a compound microscope equipped with an Olympus U-DA camera lucida (https://www.olympus-global.com). We extracted DNA from the caudal part of the causative agent (0.2 mm) using the Nucleospin Tissue kit (Macherey-Nagel, https://www.mn-net.com) according to manufacturer instructions. We performed a conventional PCR to amplify the targeted mitochondrial 12S rRNA and cox1 genes, according to instructions for specific primers and PCR conditions described elsewhere (13–15). Afterward, we sequenced the PCR products using the Applied Biosystems DNA Analyzer (ThermoFisher, https://www.thermofisher.com). We compared the 12S rDNA and cox1 sequences with public sequences in the GenBank database by using BLASTn (https://blast.ncbi.nlm.nih.gov).
We performed multiple sequence alignment of DNA sequences from the 12S rRNA and cox1 genes with ClustalW (http://www.clustal.org) using MEGA-X (https://www.megasoftware.net). We constructed phylogenetic trees using the maximum-likelihood method with 1,000 bootstrap resamplings as implemented in MEGA-X (16). We used publicly available sequences from different nematode species for comparison. We selected the Hasegawa–Kishino–Yano model as a suitable substitution model (17).
Morphologic Identification
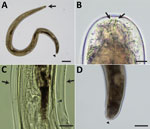
The surgically removed female nematode had a coiled body, 3.5 mm long and 198 μm wide at the midbody (Figure 2, panel A). The head was bluntly rounded and the preesophageal cuticular ring (6.3 µm wide × 2.5 µm high) was distinct (Figure 2, panel B). Labial and cephalic papillae, arranged in 4 submedian pairs, were not markedly protuberant. Amphids were small, lateral, and not salient. A slight neck was found 69 µm from the anterior extremity. The nerve ring was situated 138 µm from the anterior extremity. The esophagus was 608 µm long, and its diameter slightly widened in the posterior half. The vulva was located at the level of the posterior half of the esophagus, 395 µm from the anterior extremity. No microfilariae were present in the uteri. A postdeirid was found on the left side, 300 µm from the caudal extremity (Figure 2, panel C). Lateral alae were present, broadening toward the posterior extremity. At the level of the postdeirid, the ala was 28 µm wide. The intestine was filled with brown pigments. The cuticle was thick, 5–10 µm wide, at the postdeirid level. The tail extremity was rounded (Figure 2, panel D). We deposited the specimen (MNHN-114YT) in the Muséum National d’Histoire Naturelle, Paris, France (https://www.mnhn.fr).
Molecular Analyses
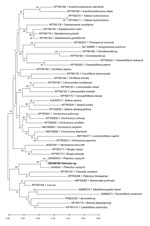
Based on the best match in BLAST, the 12S rDNA sequence (468 bp) (Figure 3) showed 95%–96% identity with P. copsychi (GenBank accession nos. OK480976 and OK480977) and the cox1 sequence (611 bp) (Figure 4) showed 92%–96% identity with P. copsychi (GenBank accession nos. OK480041 and OK480043). The calculated p-distance for cox1 sequences between this causative agent and P. copsychi was 6.71%. We submitted sequences generated in this study to GenBank (accession nos. OR346706 [cox1] and OR396900 [12S rRNA]).
The parasite specimen removed from this patient had a coiled body, rounded anterior and posterior extremities, distinct preesophageal cuticular ring, lateral alae, and a postdeirid. In addition, the vulva opened in the esophageal region. Those morphologic characteristics, along with the described morphometrics, indicated that the causative agent was a young, unmated female nematode of the genus Pelecitus (5,18). We compared that specimen with P. copsychi, P. ceylonensis, and P. galli nematodes from avian hosts in the Indomalayan realm (5,7,8). The specimen from our patient showed greater similarity to P. copsychi nematode than to the other 2 species, particularly in terms of body length. However, the esophagus of the specimen from our patient was shorter, only half the length of the P. copsychi esophagus. Although the morphometrics of microfilariae would have been necessary to differentiate species, the female nematode harbored no microfilariae. Nevertheless, we inferred that the taxonomic species of the specimen from our patient differed from P. copsychi.
Our molecular analyses positioned the specimen from our patient near P. copsychi taxonomically (Figures 3, 4). The calculated p-distance for cox1 gene sequences between the specimen from our patient and P. copsychi was 6.71%. Filarial nematodes can be considered of the same species if the genetic distance based on their cox1 sequences is <2% (19). Interspecific distances are >4.8% in filariae. A 2017 study confirmed this distance threshold in the Onchocerca species (20). Therefore, genetic distances suggested the specimen from our patient differed from P. copsychi at the species level. However, because the specimen from our patient possessed the general morphologic characteristics of Pelecitus nematodes and was positioned near P. copsychi in the phylogenetic trees, we concluded that it is a congener, currently identified only as Pelecitus sp.
Previously, 2 cases of zoonotic ocular infections possibly caused by Pelecitus spp. nematodes have been described in humans (9,10). The specimen from our patient was similar to the Pelecitus sp. nematode isolated from the iris fibers of a man in Brazil (10). The specimen from Brazil was a male worm with a preesophageal cuticular ring, lateral alae, and a postdeirid. In another case in Colombia, a male specimen of the genus Loaina was observed in the anterior chamber of the patient’s eye (9) but was later suggested to be of the genus Pelecitus (4,10). The study from Brazil (10) also stated that 2 specimens from humans in Colombia and Brazil were Pelecitus nematodes from birds. However, in the previous reports, no DNA sequences were obtained (9,10). Hence, we were unable to compare the sequences from our study with species from previous human ocular Pelecitus infections.
Several modalities in the treatment of ocular parasitic infections have been described elsewhere. Lasers, including argon, Nd:YAG, and diode, are recommended to immobilize and kill the parasite before removal (21), but surgical removal is the mainstay of treatment options (2,3). Data are lacking regarding the efficacy of available anthelmintics for the treatment of Pelecitus infections (22). In animal studies, use of ivermectin, either alone or in combination with systemic steroids, may be effective against Pelecitus infection in macaws (23). In our case-patient, the intracameral parasite was successfully removed through surgery followed by treatment of the patient with postoperative topical antimicrobials and steroids. No recurrence occurred during the 1-year follow-up period.
In conclusion, we report a case of human intraocular infection caused by a Pelecitus sp. nematode in Thailand. This finding expands the known geographic range of human infection with this zoonotic nematode, formerly reported only in South America. Guided by an initial morphologic analysis, molecular methods such as PCR can be useful for identifying rare infections such as Pelecitus sp. nematodes in humans, offering a rapid and accurate diagnostic approach. Healthcare providers should consider Pelecitus spp. nematodes as a possible causative agent in cases of small-to-moderate–sized helminths lodged in iris tissue.
Dr. Rujkorakarn is a vitreoretinal specialist at the Department of Ophthalmology, Faculty of Medicine, Mahasarakham University, Thailand. Her primary research interests are in the fields of ocular infection and inflammation, as well as retinal diseases.
Acknowledgments
We gratefully acknowledge the valuable assistance of Kerstin Junker in the preparation of this manuscript.
This research project was financially supported by Mahasarakham University.
References
- Nimir AR, Saliem A, Ibrahim IAA. Ophthalmic parasitosis: a review article. Interdiscip Perspect Infect Dis. 2012;2012:
587402 . DOIPubMedGoogle Scholar - Sinawat S, Trisakul T, Choi S, Morley M, Sinawat S, Yospaiboon Y. Ocular angiostrongyliasis in Thailand: a retrospective analysis over two decades. Clin Ophthalmol. 2019;13:1027–31. DOIPubMedGoogle Scholar
- Kongwattananon W, Wiriyabanditkul T, Supwatjariyakul W, Somkijrungroj T. Intracameral gnathostomiasis: a case report and literature review. Ocul Immunol Inflamm. 2023;31:1092–6. DOIPubMedGoogle Scholar
- Bartlett CM, Greiner EC. A revision of Pelecitus Railliet & Henry, 1910 (Filarioidea, Dirofilariinae) and evidence for the “capture” by mammals of filarioids from birds [in French]. Bull Mus Natl Hist Nat Sect A: Zool Biol Ecol Anim. 1986;8:47–99. DOIGoogle Scholar
- Uni S, Mat Udin AS, Tan PE, Rodrigues J, Martin C, Junker K, et al. Description and molecular characterisation of Pelecitus copsychi Uni, Mat Udin & Martin n. sp. (Nematoda: Onchocercidae) from the white-rumped shama Copsychus malabaricus (Scopoli) (Passeriformes: Muscicapidae) of Pahang, Malaysia. Curr Res Parasitol Vector Borne Dis. 2022;2:
100078 . DOIPubMedGoogle Scholar - Anderson RC. Nematode parasites of vertebrates: their development and transmission. 2nd ed. Wallingford (UK): CAB International; 2000.
- Dissanike AS, Fernando MA. Pelecitus galli n. sp. from the Malayan jungle fowl Gallus gallus spadiceus. J Helminthol. 1974;48:199–203. DOIPubMedGoogle Scholar
- Dissanike AS. Pelecitus ceylonensis n. sp., from the chick and ash-dove experimentally infected with larvae from Mansonia crassipes, and from naturally-infected crows in Ceylon. Ceylon J Sci. 1967;7:96–104.
- Botero D, Aguledo LM, Uribe FJ, Esslinger JH, Beaver PC. Intraocular filaria, a Loaina species, from man in Colombia. Am J Trop Med Hyg. 1984;33:578–82. DOIPubMedGoogle Scholar
- Bain O, Otranto D, Diniz DG, dos Santos JN, de Oliveira NP, Frota de Almeida IN, et al. Human intraocular filariasis caused by Pelecitus sp. nematode, Brazil. Emerg Infect Dis. 2011;17:867–9. DOIPubMedGoogle Scholar
- Chan AHE, Chaisiri K, Morand S, Saralamba N, Thaenkham U. Evaluation and utility of mitochondrial ribosomal genes for molecular systematics of parasitic nematodes. Parasit Vectors. 2020;13:364. DOIPubMedGoogle Scholar
- Chan AHE, Chaisiri K, Dusitsittipon S, Jakkul W, Charoennitiwat V, Komalamisra C, et al. Mitochondrial ribosomal genes as novel genetic markers for discrimination of closely related species in the Angiostrongylus cantonensis lineage. Acta Trop. 2020;211:
105645 . DOIPubMedGoogle Scholar - Casiraghi M, Anderson TJ, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. DOIPubMedGoogle Scholar
- Casiraghi M, Bain O, Guerrero R, Martin C, Pocacqua V, Gardner SL, et al. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int J Parasitol. 2004;34:191–203. DOIPubMedGoogle Scholar
- Sobotyk C, Foster T, Callahan RT, McLean NJ, Verocai GG. Zoonotic Thelazia californiensis in dogs from New Mexico, USA, and a review of North American cases in animals and humans. Vet Parasitol Reg Stud Reports. 2021;24:
100553 . DOIPubMedGoogle Scholar - Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. DOIPubMedGoogle Scholar
- Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–74. DOIPubMedGoogle Scholar
- Gibbons LM. Keys to the nematode parasites of vertebrates. Supplementary volume. Wallingford (UK): CAB International; 2010.
- Ferri E, Barbuto M, Bain O, Galimberti A, Uni S, Guerrero R, et al. Integrated taxonomy: traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda). Front Zool. 2009;6:1. DOIPubMedGoogle Scholar
- Lefoulon E, Giannelli A, Makepeace BL, Mutafchiev Y, Townson S, Uni S, et al. Whence river blindness? The domestication of mammals and host-parasite co-evolution in the nematode genus Onchocerca. Int J Parasitol. 2017;47:457–70. DOIPubMedGoogle Scholar
- Murugan SB. Commentary: Angiostrongylus cantonensis in anterior chamber. Indian J Ophthalmol. 2019;67:161–2. DOIPubMedGoogle Scholar
- Morishita TY, Schaul JC. Parasites of birds. In: Baker DG, editor. Flynn’s parasites of laboratory animals. Ames (IA): Blackwell Publishing; 2007. p. 217–301.
- Allen JL, Kollias GV, Greiner EC, Boyce W. Subcutaneous filariasis (Pelecitus sp.) in a yellow-collared macaw (Ara auricollis). Avian Dis. 1985;29:891–4. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: August 09, 2024
Table of Contents – Volume 30, Number 9—September 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Pukkapol Suvannachart, 296 Nakhornsawan Rd, Department of Ophthalmology, Suddhavej Hospital, Faculty of Medicine, Mahasarakham University, Maha Sarakham 44000, Thailand
Top