Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Detection of SARS-CoV-2 Reinfections Using Nucleocapsid Antibody Boosting
Eduard Grebe
, Daniel Chacreton, Mars Stone, Bryan R. Spencer, James Haynes, Akintunde Akinseye, Marion C. Lanteri, Valerie Green, Hasan Sulaeman, Roberta Bruhn, Vivian I. Avelino-Silva, Paul Contestable, Brad J. Biggerstaff, Melissa M. Coughlin, Brian Custer, Jefferson M. Jones, David Wright
1, and Michael P. Busch
1
Author affiliation: Vitalant Research Institute, San Francisco, California, USA (E. Grebe, M. Stone, H. Sulaeman, R. Bruhn, V.I. Avelino-Silva, B. Custer, M.P. Busch); Westat, Rockville, Maryland, USA (D. Chacreton, A. Akinseye, D. Wright); University of California San Francisco, San Francisco (M. Stone, R. Bruhn, B. Custer, M.P. Busch); American Red Cross, Dedham, Massachusetts, USA (B.R. Spencer); American Red Cross, Rockville (J.M. Haynes); Creative Testing Solutions, Tempe, Arizona, USA (M.C. Lanteri, V. Green); San Francisco State University, San Francisco (H. Sulaeman); QuidelOrtho, Rochester, New York, USA (P. Contestable); Centers for Disease Control and Prevention, Fort Collins, Colorado, USA (B.J. Biggerstaff); Centers for Disease Control and Prevention, Atlanta, Georgia, USA (M.M. Coughlin, J.M. Jones)
Main Article
Figure 1
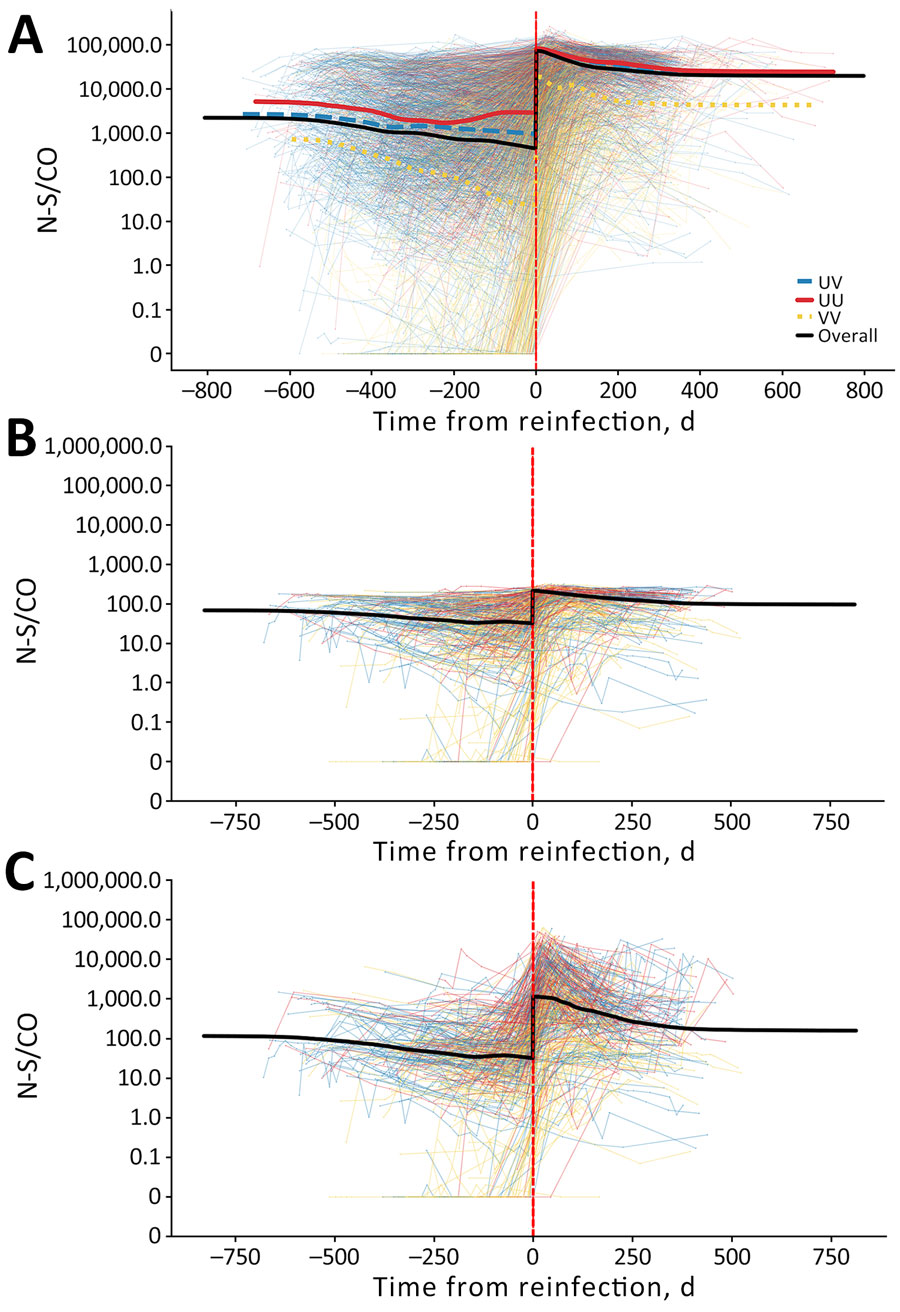
Figure 1. Individual anti-N S/CO trajectories before and after swab-confirmed reinfection in vaccinated and unvaccinated participants in study of detection of SARS-CoV-2 reinfections using nucleocapsid antibody boosting. A) S/CO trajectories with neat-only anti-N testing of all donors with reinfections in the study. B) Trajectories of test results from 434 donors with reinfections subjected to expanded dynamic range dilutional anti-N testing; neat results only. C) Trajectories of test results from the same 434 donors with reinfections subjected to dilutional anti-N testing; dilutional (expanded dynamic range) testing results only. Images show average anti-N trajectories of donors who experienced reinfections, with and without expanded dynamic range testing, stratified by vaccination status. Time represents days before or after swab-confirmed reinfection (vertical red dashed line). N, nucleocapsid; S/CO, signal-to-cutoff ratio; UU, unvaccinated at the time of first infection and reinfection; UV, unvaccinated at first infection and vaccinated at reinfection; VV, vaccinated at first infection and reinfection.
Main Article
Page created: March 25, 2025
Page updated: April 22, 2025
Page reviewed: April 22, 2025
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
