Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 31, Number 5—May 2025
Research Letter
Trichophyton indotineae Infection, São Paulo, Brazil, 2024
Suggested citation for this article
Abstract
We report an extensive, terbinafine-resistant (squalene epoxidase F397L mutation) Trichophyton indotineae infection in a previously healthy businessman from São Paulo, Brazil. The patient had previously traveled to France, Spain, and the United States. Clinician awareness, laboratory testing capacity, and surveillance are essential to prevent T. indotineae spread and inform healthcare practices.
Trichophyton indotineae is an anthropophilic, frequently terbinafine-resistant fungus causing recalcitrant dermatophytosis. It has become endemic in South Asia; cases are documented across 6 continents, and possible local US transmission has been reported (1,2). São Paulo, Brazil, South America’s largest city, is known for its global business connections and frequent international travelers.
In September 2024, a previously healthy São Paulo man in his 40s sought treatment for difficult-to-treat tinea cruris. In October 2023, he traveled to Paris and Barcelona, and 30 days later, he traveled to Boston, Massachusetts, USA. He had not traveled to Asia. Six weeks after he returned home, he noticed pruritic, erythematous, bilateral groin lesions. He treated the lesions with topical betamethasone and ketoconazole, but they worsened. After consulting multiple dermatologists, he was prescribed oral terbinafine (9 weeks), with no improvement.
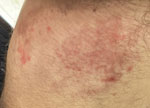
Subsequently, another dermatologist, accessed by telemedicine, performed a full-body examination and identified additional lesions at the dorsal region of the left foot. Groin lesions had irregular borders, erythematous inflammatory areas, and reddish scaly plaques (Figure 1); the dorsal region of the left foot had small scaly erythematous plaques. Direct microscopy from groin and foot skin scrapings was positive, and a culture of groin scrapings showed dermatophyte mold.
Initial matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis using the Biotyper 3.0 database (Bruker Daltonics, https://www.bruker.com) identified the organism as part of the Trichophyton mentagrophytes group. Additional analysis using the MSI-2 database (Université Paris VI, https://msi.happy-dev.fr) identified T. indotineae with high confidence (3). The physician switched the patient’s treatment to itraconazole (200 mg/d), with substantial improvement noted after 8 weeks.
We confirmed the identification of T. indotineae by using internal transcribed spacer ribosomal DNA sequencing analysis (GenBank accession no. PQ726960). Antifungal susceptibility testing (Appendix) for the isolate using broth microdilution showed high MICs against terbinafine (>4 mg/L) and fluconazole (32 mg/L) and strong in vitro activity against itraconazole (0.016 mg/L) and voriconazole (0.125 mg/L) (4). Currently, clinical breakpoints for interpreting antifungal susceptibility testing of T. indotineae do not exist.
We performed genomic analyses to assess the isolate’s possible origins and to detect the presence of squalene epoxidase (SQLE) gene mutations associated with terbinafine resistance (5,6). We performed whole-genome sequencing by using the NextSeq 550 system (Illumina, https://www.illumina.com). We deposited read data into National Center for Biotechnology Institute Sequence Read Archive database (https://www.ncbi.nlm.nih.gov/sra; BioProject no. PRJNA1196410). We downloaded an additional 347 T. indotineae sequences from isolates collected in 14 countries from the Sequence Read Archive database (Appendix Table 2) and included in the genomic analysis (A.R. dos Santos et al., unpub. data). We performed single-nucleotide polymorphism (SNP) identification and phylogenetic analysis using MycoSNP version 1.5 (https://github.com/CDCgov/mycosnp-nf), with T. indotineae strain TIMM20114 as the reference genome. We performed SNP identification in the SQLE gene by mapping filtered reads to a reference sequence (OM313310.1) by using the Burrows-Wheeler Aligner (https://bio-bwa.sourceforge.net), followed by variant calling with freebayes (https://github.com/freebayes/freebayes). Genomic analysis showed that the isolate from the patient from Brazil was closely related to other terbinafine-resistant T. indotineae isolates from 13 countries (Figure 2). Among isolates analyzed, the isolate from the patient from Brazil was most genetically similar to one from Lower Saxony, Germany (21 SNPs distance). The isolate was collected from a patient with terbinafine-resistant T. indotineae in June 2022, and, like the isolate from the patient in Brazil, it had a terbinafine resistance–conferring SQLE gene mutation (F397L).
We report a case of tinea caused by terbinafine-resistant T. indotineae in a businessman from Brazil who traveled to Europe and the United States. Genomic analysis revealed that the patient’s isolate contained a terbinafine resistance–conferring SQLE mutation, F397L, and fit within a predominantly terbinafine-resistant cluster of isolates collected from countries across North America, Europe, and Asia. Although it is uncertain where the patient acquired infection, his isolate’s close relatedness to one from Germany suggests possible acquisition in Europe. However, additional analysis, including of isolates from Barcelona, Paris, and Boston, is essential to confirm where the infection was acquired.
Clinicians should be vigilant for possible T. indotineae infection in persons who have traveled abroad or seek treatment for difficult-to-treat tinea because local transmission may occur. Clinicians should advise those patients about strategies to prevent transmission (7), need for prolonged therapy (e.g., >6 weeks) with itraconazole (8), and importance of avoiding topical corticosteroids and antifungal corticosteroid products, which can worsen tinea infections (8).
In Brazil and other resource-limited settings, lack of specialized and well-equipped microbiology laboratories could enable unrecognized introduction and local spread (9). Increasing laboratory capacity for dermatophyte species identification, antifungal susceptibility testing, and genomic epidemiology studies is essential for monitoring transmission patterns and guiding effective treatment strategies (10). Combining matrix-assisted laser desorption/ionization time-of-flight mass spectrometry with the improved MSI-2 database might help overcome challenges of identifying T. indotineae in skin scraping cultures in clinical laboratories. That approach might also enhance epidemiologic understanding of global spread of that species and contribute to improved patient care (3). In conclusion, this case highlights the importance of integrating clinical, microbiological, and genomic data to address spread of antimicrobial-resistant pathogens in an increasingly interconnected world.
Dr. Nobrega de Almeida Jr is a physician specializing in infectious diseases and clinical pathology. He serves as a medical assistant at the Clinical Laboratory of Hospital Albert Einstein in São Paulo and is an assistant professor of infectious diseases at the Federal University of São Paulo. His primary research interest is in medical mycology.
Acknowledgments
We thank the whole Mycology and Molecular Biology Teams from the Clinical Laboratory of Hospital Albert Einstein, including Wendy Colalto Rodrigues da Silva, Kornelia Bogner, Lidiane de Oliveria, Fabiane Camargo Gomes Nunes, Cristiani da Silva Calo, Nair Hideko Muto, and Rubia Anita Ferraz Santana, for invaluable support in sample processing, data analysis, and expertise in molecular techniques that contributed to the success of this study.
This report was approved by the institutional review board from Hospital Albert Einstein (no. CAAE 85377824.2.0000.0071). J.N.d.A. has a research grant from Fundação de Amparo à Pesquisa do Estado de São Paulo (grant no. 2024/12605-9).
References
- Uhrlaß S, Verma SB, Gräser Y, Rezaei-Matehkolaei A, Hatami M, Schaller M, et al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. DOIPubMedGoogle Scholar
- Caplan AS, Todd GC, Zhu Y, Sikora M, Akoh CC, Jakus J, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. 2024;160:701–9. DOIPubMedGoogle Scholar
- Normand AC, Moreno-Sabater A, Jabet A, Hamane S, Cremer G, Foulet F, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. DOIPubMedGoogle Scholar
- Arendrup MC, Kahlmeter G, Guinea J, Meletiadis J; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). How to: perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E.Def 11.0, exemplified by Trichophyton. Clin Microbiol Infect. 2021;27:55–60. DOIPubMedGoogle Scholar
- De Paepe R, Normand AC, Uhrlaß S, Nenoff P, Piarroux R, Packeu A. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. DOIPubMedGoogle Scholar
- Saunte DML, Hare RK, Jørgensen KM, Jørgensen R, Deleuran M, Zachariae CO, et al. Emerging terbinafine resistance in Trichophyton: clinical characteristics, squalene epoxidase gene mutations, and a reliable EUCAST method for detection. Antimicrob Agents Chemother. 2019;63:e01126–219. DOIPubMedGoogle Scholar
- American Academy of Dermatology Association. Preventing and treating Trichophyton indotineae [cited 2024 Dec 17]. https://www.aad.org/member/clinical-quality/clinical-care/emerging-diseases/dermatophytes/preventing-treating-trichophyton-indotineae
- Khurana A, Sharath S, Sardana K, Chowdhary A. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J Am Acad Dermatol. 2024;91:315–23. DOIPubMedGoogle Scholar
- Falci DR, Pasqualotto AC. Clinical mycology in Latin America and the Caribbean: A snapshot of diagnostic and therapeutic capabilities. Mycoses. 2019;62:368–73. DOIPubMedGoogle Scholar
- Lockhart SR, Smith DJ, Gold JAW. Trichophyton indotineae and other terbinafine-resistant dermatophytes in North America. J Clin Microbiol. 2023;61:
e0090323 . DOIPubMedGoogle Scholar
Figures
Suggested citation for this article: Nobrega de Almeida Jr J, dos Santos AR, de S. Trindade MR, Gold JAW, Razo FPM, Gonçalves SS, et al. Trichophyton indotineae infection, São Paulo, Brazil, 2024. Emerg Infect Dis. 2025 May [date cited]. https://doi.org/10.3201/eid3105.250048
Original Publication Date: April 22, 2025
Table of Contents – Volume 31, Number 5—May 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
João Nobrega de Almeida Jr, Hospital Israelita Albert Einstein, Av Albert Einstein, 627/701 Morumbi, São Paulo SP 05652–900, Brazil
Top