Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Volume 31, Number 5—May 2025
Dispatch
Tropheryma whipplei Infections, Mexico, 2019–2021
Suggested citation for this article
Abstract
Whipple’s disease is rarely diagnosed in Latin America. We describe 2 patients with Tropheryma whipplei infection diagnosed in Mexico during 2019–2021. Diagnoses were confirmed by histopathology, electron microscopy, immunohistochemistry and DNA amplification and sequencing analysis of the 16S rRNA gene. Clinicians should be aware of T. whipplei infection and associated syndromes.
Whipple’s disease (WD) is an unusual infection with protean manifestations caused by Tropheryma whipplei, a fastidious, slow-growing, gram-positive bacterium. Classical WD was originally described in 1907 and is characterized by polyarthritis, diarrhea, and lymphadenopathy (1). WD is a rare disease with a prevalence of 9.8 cases/1 million inhabitants in the United States and is most frequently identified in White men >50 years of age (2). WD has also been described among patients with various immunosuppressive conditions, including persons with HIV infection (3,4).
In the appropriate clinical context, WD is suspected when abundant foamy macrophages containing abundant periodic acid–Schiff stain (PAS)–positive and diastase-resistant granules are found in the lamina propria of small bowel biopsy specimens (5). However, WD can also be documented as a localized disease in cases of endocarditis, uveitis, isolated lymphadenopathy, encephalitis, or arthritis and negative cultures (5).
Classic WD has been reported extensively in western Europe, Canada, and in the United States (6). The disease has been described only rarely in countries in Latin America, including a probable case in Mexico (7–10). The paucity of descriptions of WD from those countries could relate to underrecognition, limited access to confirmatory diagnostic assays, or genetic characteristics of the predominant population in healthcare settings. We report 2 patients with classic WD from the states of Veracruz and Mexico City in Mexico.
The first patient was a previously healthy 63-year-old man whose signs and symptoms began 2 years earlier and included cough, pneumonia, intermittent diarrhea, and weight loss of 42 kg. Results of serologic tests for celiac disease and stool bacterial cultures and ova and parasite exams were all negative. An echocardiogram revealed moderate aortic valve insufficiency and stenosis, along with severe mitral valve insufficiency, a dilated left ventricle, and mild pulmonary arterial hypertension. Small bowel endoscopy revealed multiple white nodules in the duodenum and ileum. Small bowel biopsies revealed abundant macrophages with foamy, pale, blue-gray cytoplasm in the lamina propria (Figure 1, panel A), which contained numerous globular and falciform inclusions that were strongly PAS-positive and diastase-resistant (Figure 1, panel B). Immunohistochemistry results for T. whipplei (8) were positive (Figure 1, panel C), and the PCR for gram-positive–specific 16S rRNA V8/V9 gene (11) produced a target 350-bp amplicon. After Sanger sequencing, Tropheryma spp. was identified through BLAST search (https://blast.ncbi.nlm.nih.gov). The patient initially received ceftriaxone, then received combination doxycycline and hydroxychloroquine therapy. One year after diagnosis, he had another round of duodenum and ileum biopsies, which revealed persistence of macrophages with PAS-positive inclusions; no additional studies were done. Combination therapy with doxycycline and hydroxychloroquine was reinitiated and, 2 years after those biopsies, the patient remained asymptomatic. No further endoscopic procedures were conducted.
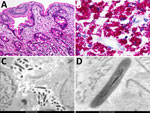
The second patient was a 45-year-old man with well-controlled HIV infection. He was receiving raltegravir, darunavir, etravirine, and ritonavir, and his viral load was undetectable. His CD4 count was 439 cells/mm3. He had a history of 3 months of watery diarrhea. He received ciprofloxacin for 7 days and temporarily improved; however, his diarrhea soon returned after cessation of antibiotic therapy, and then he experienced a 4 kg weight loss. Endoscopy revealed multiple white nodular areas in the mucosa of the duodenum and ileum. Biopsy specimens from both sites revealed expansion of the lamina propria by foamy macrophages with PAS-positive, diastase-resistant inclusions (Figure 2, panels A, B). Electron microscopy performed on formalin-fixed, paraffin-embedded tissue from the small bowel (duodenum and ileum) identified intracellular and extracellular bacilli with a trilaminar plasma membrane (Figure 2, panels C, D). Molecular identification by 16S rRNA V1/V2 sequencing was performed by the Center for Advanced Molecular Diagnostics at the Brigham and Women’s Hospital (Boston, MA, USA), as previously described (12). A 321-bp contig was assembled and fed into the 16S RipSeq Single database (Pathogenomix, https://www.pathogenomix.com) and matched to T. whipplei (GenBank accession no. AF190688). We initiated doxycycline and hydroxychloroquine therapy, and his signs and symptoms resolved.
We document 2 cases of WD in 2 states in Mexico. Both patients were born and raised in the country, had small bowel biopsies histologically compatible with WD, and had T. whipplei infection confirmed by immunohistochemical staining and molecular diagnostics.
Identification of T. whipplei has epidemiologic implications because of its potential for transmission between humans through saliva or feces (5). In addition, from a therapeutic perspective, WD generally has excellent response to antibiotic regimens; however, if such regimens are not provided, WD can result in life-threatening complications (13). Because classic WD frequently involves joints, it can be misdiagnosed as autoimmune rheumatologic disease, resulting in immunosuppressive therapies that paradoxically can accelerate the course of WD (14).
In conclusion, our findings highlight the need for more specific and widely available diagnostic tests for T. whipplei in Mexico and across Latin America, particularly molecular diagnostic assays, to enable characterization of T. whipplei in the region. Clinicians should consider T. whipplei infection in the differential diagnosis of patients with debilitating, otherwise unexplained, yet treatable conditions such as malabsorption syndromes and localized disease, including culture-negative endocarditis, encephalitis, uveitis, lymphadenopathies, and inflammatory spondyloarthropathies (5,15).
Dr. Delgado-de la Mora is a gastrointestinal pathologist currently working as research assistant at Weill-Cornell Medicine, New York City. His research interests include emerging infectious diseases and viral oncogenesis.
Acknowledgment
We dedicate this article to the memory of our beloved friend and colleague, Braulio Martínez-Benítez.
References
- Whipple GH. A hitherto undescribed disease characterized anatomically by deposits of fat and fatty acids in the intestinal and mesenteric lymphatic tissues. Bull Johns Hopkins Hosp. 1907;18:382–93.
- Elchert JA, Mansoor E, Abou-Saleh M, Cooper GS. Epidemiology of Whipple’s disease in the USA between 2012 and 2017: a population-based national study. Dig Dis Sci. 2019;64:1305–11. DOIPubMedGoogle Scholar
- Lozupone C, Cota-Gomez A, Palmer BE, Linderman DJ, Charlson ES, Sodergren E, et al.; Lung HIV Microbiome Project. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med. 2013;187:1110–7. DOIPubMedGoogle Scholar
- Guérin A, Kerner G, Marr N, Markle JG, Fenollar F, Wong N, et al. IRF4 haploinsufficiency in a family with Whipple’s disease. eLife. 2018;7:
e32340 . DOIPubMedGoogle Scholar - Paddock CD, Fenollar F, Lagier JC, Raoult DA. 21st Century appraisal of Whipple’s disease and Tropheryma whipplei. Clin Microbiol Newsl. 2022;44:123–9. DOIGoogle Scholar
- Dolmans RA, Boel CH, Lacle MM, Kusters JG. Clinical manifestations, treatment, and diagnosis of Tropheryma whipplei infections. Clin Microbiol Rev. 2017;30:529–55. DOIPubMedGoogle Scholar
- Vega-López CA, Carrillo-Ocampo JL, Castillo-García J, González-de la Mora JJ, Alemán-Ortiz G, Romo-Aguirre C. [Whipple’s disease: a case report and review of the literature] [in Spanish]. Rev Gastroenterol Mex. 2010;75:517–21.PubMedGoogle Scholar
- Renon VP, Appel-da-Silva MC, D’Incao RB, Lul RM, Kirschnick LS, Galperim B. Whipple’s disease: rare disorder and late diagnosis. Rev Inst Med Trop São Paulo. 2012;54:293–7. DOIPubMedGoogle Scholar
- Rollin DC, Paddock CD, Pritt BS, Cunningham SA, Denison AM. Genotypic analysis of Tropheryma whipplei from patients with Whipple disease in the Americas. J Clin Pathol. 2017;70:891–5. DOIPubMedGoogle Scholar
- Rey R M, Orozco LA, Marrugo K, López R, Pérez-Riveros ED, De la Hoz-Valle J, et al. Whipple disease diagnosed by enteroscopy: first case report in Colombia of an underdiagnosed disease and literature review. BMC Gastroenterol. 2020;20:197. DOIPubMedGoogle Scholar
- Klausegger A, Hell M, Berger A, Zinober K, Baier S, Jones N, et al. Gram type-specific broad-range PCR amplification for rapid detection of 62 pathogenic bacteria. J Clin Microbiol. 1999;37:464–6. DOIPubMedGoogle Scholar
- Solomon IH, Lin C, Horback KL, Kanjilal S, Rojas-Rudilla V, Brigl M, et al. Utility of histologic and histochemical screening for 16S ribosomal RNA gene sequencing of formalin-fixed, paraffin-embedded tissue for bacterial endocarditis. Am J Clin Pathol. 2019;152:431–7. DOIPubMedGoogle Scholar
- Moos V, Schneider T. Changing paradigms in Whipple’s disease and infection with Tropheryma whipplei. Eur J Clin Microbiol Infect Dis. 2011;30:1151–8. DOIPubMedGoogle Scholar
- Lagier JC, Lepidi H, Raoult D, Fenollar F. Systemic Tropheryma whipplei: clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine (Baltimore). 2010;89:337–45. DOIPubMedGoogle Scholar
- Fenollar F, Lagier JC, Raoult D. Tropheryma whipplei and Whipple’s disease. J Infect. 2014;69:103–12. DOIPubMedGoogle Scholar
Figures
Suggested citation for this article: Delgado-de la Mora J, Grube-Pagola P, Paddock CD, DeLeon-Carnes M, Laga AC, Solomon IH, et al. Tropheryma whipplei infections, Mexico, 2019–2021. Emerg Infect Dis. 2025 May [date cited]. https://doi.org/10.3201/eid3105.241046
Original Publication Date: April 22, 2025
1These authors contributed equally to this article.
Table of Contents – Volume 31, Number 5—May 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jesus Delgado-de la Mora, Weill Cornell Medicine Ringgold Standard Institution, Department of Pathology and Laboratory Medicine, 1300 York Ave, New York, NY 10065, USA
Top